NL Journal of Agriculture and Biotechnology
(ISSN: 3048-9679)
Removal of Zn (II) and Cd Heavy Metal Ions from Wastewater using Modified Natural Adsorption Cattle (Verbascum Cheiranthifolium)
Author(s) : Bahdisen Gezer, Rahsen Gezer, Can Gezer, Mehmet Fazıl Coskun. DOI : 10.71168/NAB.01.01.102
Abstract
The study also investigated the adsorption of Zn (II) and Cd ions dissolved in water using the chemically modified vegetable material of Verbascum cheiranthifolium and the effect of the modification process in increasing the absorption capacity in the removal of these heavy metal ions. Before and after adsorption with pollutant metals by SEM, FTIR, and XRD methods, the surface structure and morphology (constructive properties) of adsorbents and the adsorbing mechanisms of polluting metals have been determined. The modification was done with a solution of sulfuric acid. Further, kinetic, isothermic, and thermodynamic studies have been carried out on bovine plant material modified with sulfuric acid. The chemical oxygen requirement for using raw cattle plant material in the water ranges from 930 to 1440 mg/L, while this value has dropped to 10-50 mg/L in modified adsorbents. The yields varied with the concentration of heavy metals, but modified cattle feed was found to be around 96% for plant material. The maximum adsorption capacity of heavy metals was found to be around 614 mg/g. Regeneration studies were also carried out to investigate the reusability of cattle feed plant material after the adsorption process. As a result, the capacity of low-cost adsorbents is economically utilizable for removing Zn (II) and Cd from wastewater by adsorption in low hydration.
Introduction
In recent years, accelerated population growth, rapid urbanization, excessive human consumption, and rapidly evolving industrial developments have led to environmental pollution and water resources being rapidly polluted by various pollutants. Wastes that cause pollution (which may have different physical properties) are pollutants that can disrupt the physico-chemical and biochemical properties of the environment in which they enter. One of the environmental problems that threaten humanity’s future is water pollution [1].
Together with the hydrological circuit, pollutants can reach from surface water to groundwater. Household, agricultural and industrial waste that reaches wetlands without treatment or with insufficient treatment pollutes drinking and drinking water sources along with access to groundwater, causing water pollution [2]. Waste from industries differs greatly in source, quantity, and character from domestic waste. The effects of industrial waste on nature are far more significant, altering the natural balance and, in some cases, irreversible [3]. Therefore, effective treatment is a prerequisite for ensuring the reuse of water and discharge standards to the receiving environments. Water pollution sources are diverse, and metals play a major role in natural and anthropogenic contamination of water. These pollutants can have chronic toxicity and carcinogenic effects [4]. These wastes are classified as hazardous and harmful waste. Among those in this class, heavy metals are the primary source of toxicity [5]. Heavy metals (such as Ag, As, Cd, Cu, Fe, Hg, Ni, Pb, Zn, etc.) [6]. Annually, 332,000 tonnes of lead resulting from natural cycles are released to the atmosphere, with 18,800 tonnes of arsenic, 7,600 tonnes of Cd [7] and 3600 tonnes of mercury being discharged, while the quantities released as a result of human activities are to be three times higher than those of selenium (19 times), cadmium (8 times), mercury, lead, lead (6 times), arsenics, nickel and chromium (3 times) [8]. These data show that human-influenced transmission is more than natural transmission.
The rise in heavy metal pollution as civilization develops poses a threat to all living things, especially humans. That is why restrictive regulations have been introduced on levels of painful metals in agricultural land, nutrients, and even wastewater, primarily in sources of drinking water. The method should be determined according to the area where the pollution will be removed, as well as the method of removal according to metal type and characteristics. Of the known methods, the cheap and convenient ones are preferred. Studies are being undertaken to increase the capacity to use methods such as decay, ion exchange, adsorption, electrodialysis, etc [3].
The most widely used alternative treatment method in recent years is the adsorption method, which is based on the accumulation of substances dissolved in the solution on a suitable intermediate surface (adsorbent) [9]. Although the preferred adsorbents in this method are of natural origin, organic wastes that have been separated at the source for the highest efficiency of treatment are still being improved in terms of their absorbance properties through various processes [10].
The aim of this study is to improve the adsorption capacity of heavy metals in wastewater in the use of cattle plant material as an adsorbent through the process of modification. It is also intended to find kinetic and thermodynamic parameters for this adsorbent in adsorption processes.
Materials and Methods
Adsorption Material
The cattle tail (Verbascum cheiranthifolium) plant was collected from the grove and surrounding area and held for drying in a dark room for 10 days (Figure 1). Later, the drying plants (Figure 2), were split into small particles. Dried samples in small pieces were ground in the coffee mill to make them smaller. It was split into dimensions in 20 minutes in the Electromagnetic Digital Elevator. Samples of 20μm were used for adsorption experiments. The samples separated after processing were dried for 24 hours at 90°C. Subsequently, the beef samples were kept closed until they were used in containers with lids so that they were not affected by environmental conditions.

Figure 1. The cattle tail (Verbascum cheiranthifolium) plant was used in the study.

Figure 2. The state of the cattle tail (Verbascum cheiranthifolium) plant after it is collected and dried.
Chemicals and tools used
1 NHCl and 1 N NaOH were used for pH adjustment of adsorption solutions, H2SO4 for processes of modification of cattle tail plant material. All chemicals are analytically pure. Bandelin RK 255H Ultrasonic Bath was used for adsorption experiments. It was done using the ICP device and the UV-Vis spectroscopic photometer for heavy metal designations in pre- and post-adsorption solutions. The samples were analyzed using the Fourier Transform Infrared Spectrometer, a solid-phase infrared spectrum, the Scanned Electron Microscope (SEM), and the FTIR spectrometer (Nicolet 380 (Thermo Scientific), to observe and compare the molecular structures of raw and modified materials.
Modification processes
The modification process was carried out in an acidic environment to increase the active surfaces of the plant material Verbascum cheiranthifolium and to remove the parts that color the water. To do this, the adsorbent was first washed with pure water three times and dried at 100oC in the shell to remove any foreign substances attached to the surface and substances consisting of water-soluble compounds. For modification, 250 ml solvents were processed for 24 hours at 30oC temperature and 35 kHz frequency with a 2 g bovine plant sample for 0.5, 1.0, 1.5, 2.0, and 2.5 N H2SO4 solution. Then the solutions were filtered. The pH of the samples was washed with pure water 5 times to reach a neutral level [7].
Isotherm Calculations
In experiments, different pH, adsorbent dose, solution temperature, mixing speed, contact time and heavy metal concentrations were studied. The metal concentrations of the solutions were analyzed before the contact period (Co) and after (Ce). The following equation has been used in the calculation of yield;
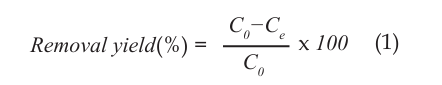
The data obtained were applied to the linearized isomers of Langmuir [11], Freundlich [11], and Dubinin-Radushkevich [12]. Linearize Langmuir, Freundlich, and D-R isoters are given in equations (2), (3), and (4).
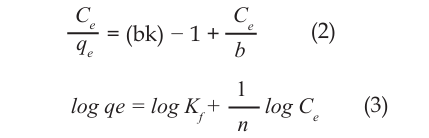
Here, the quantity of metal held per unit adsorbent weight at the system balance (mg g-1), the volume of the solution V (L), the amount of the absorbance M (g), the concentration of the metal in Ce balance conditions (mg L–1), b (mg G–1) adsorption capacity, and the Langmuir constants related to the energy of the absorption K (L mg–1), Kf and 1/n Freundlich constants are expressed [13].
Adsorption experiments
To investigate the dissolving properties of natural adsorbents, working conditions have been applied in experiments, such as pH 2, 4, 6, 8, and 10, and contact times at temperatures 20, 25, 30, 35, and 45 C, 10, 30, 60, 90 and 120 min.
In collapse experiments, Zn Cd, with an initial concentration of 20 mg/L, was mixed with metal ions, increasing the pH with NaOH for 30 minutes. After mixing, a sample of the clear solution on which the solution was left to rest for 10 minutes was taken and the metal concentration remaining in the solution analyzed so that the quantities of collapse and metal waste were determined. In adsorption experiments, metal ions at 20 mg/L concentration were combined with the adsorbent at 5 g/L and mixed in an ultrasonic bath for 45 minutes. The concentration of metal remaining in the solution was analyzed by filtering. The results were evaluated by calculating the yield (%) and the concentration of the adsorbed metal on the solid, qm (mg/g) [14]. The calculation of these data is given in equations 1 and 2:
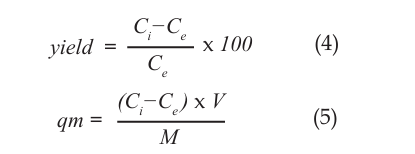
Here; Ci: indicates the initial adsorbent concentration (mg/L), Ce: is the concentration remaining in the solution at the end of the experiment (mg / L), V: the volume of the solution (L), M: is the amount of the adsorbing agent (gr).
Results and Discussion
To determine the factors that will produce the best yield of the material in environmental conditions, a five-faceted situation has been chosen in the literature of each factor for determining the state that will yield the greatest yield in different combinations of these conditions and the estimated value. The experiments were repeated at 5 different conditions around the best yield. The factors for eliminating the highest amount of metal contamination per unit of adsorbent have been identified. Thus, experiments were carried out for data to be used in kinetic calculations for the best cost yield. For Zn (II), the metal solution has a pH of 4.0, a temperature of 5oC, a contact time of 30 minutes, and a metal solution of 6oC for Cd.
SEM images taken after raw and modified cattle plant material are shown in Figure 3. It is seen here that the cattle plant material has a large surface structure. It was also revealed in the SEM image that the surface structure of cattle plant material consists of thin hair-shaped structures, and that the modification and the increased thin-hair-shape structures. This property has been identified as an important feature that increases the adsorption capacity [15].
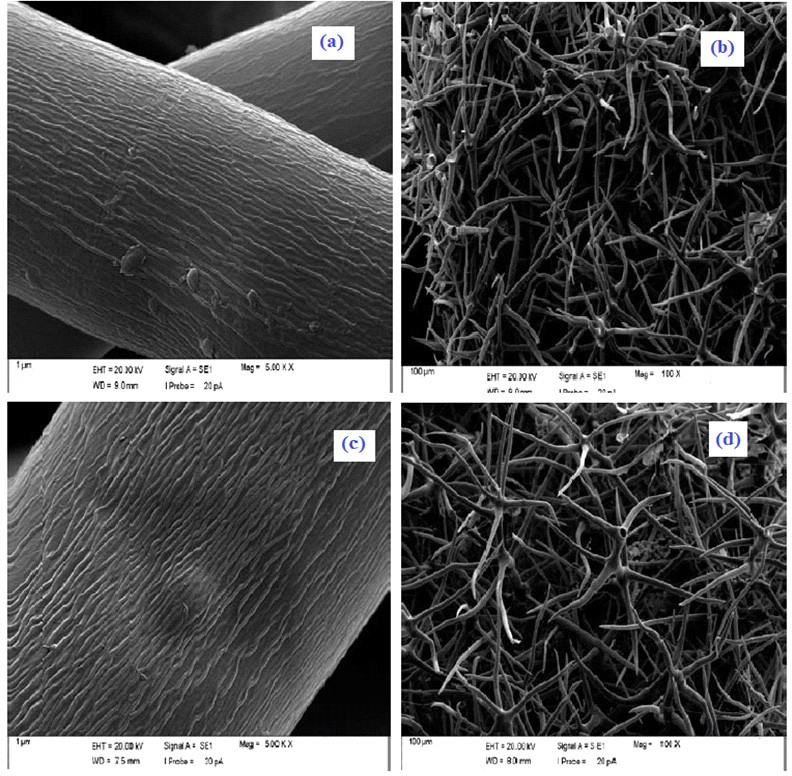
Figure 3. For heavy metal adsorption, SEM images of (a) and (b) raw cattle tail plant material resulting from the process of modification of (c) or (d).
Before and after the modifications, the FTIR spectrums of cattle feed plant material are given in Figure 4. Looking at the FTIR spectrum, it is clear that the chemical processes resulted in the conversion of cattle feed into very few structures. Based on literature information, the FTIR spectrum of cattle plant material gives the aliphatic structures PTIR spikes around 2950- 2840 cm-1 wavelengths, while the aromatic hydrocarbon spikes appear around 3030 cm-1, 1500 cm-1 and 1490 cm-1 [16]. The 1700 cm-1 wavelength bond is associated with the extension of C-O in the linear aliphatic aldehyde, ketones, and carboxyls present in the initial cane. The bonds between 980 cm-1 and 1370 cm-1 indicate C-O bonds [18] while the weak bonds from 3400-3560 cm-1 represent -NH and -OH groups. The 3450 cm-1 wavelength spikes have been identified as alcohol and phenol spikes. Aromatic spikes increase density as they increase the temperature of modification [19].
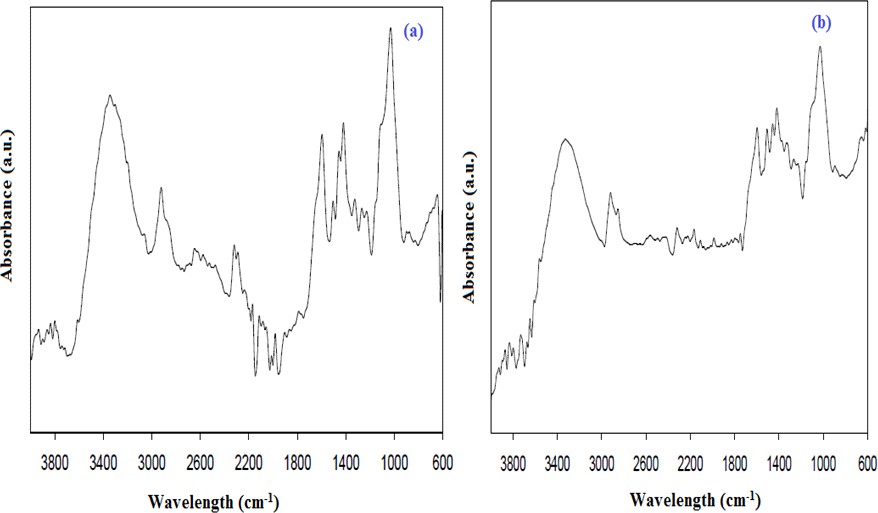
Figure 4. (a) Raw cattle feed, (b) FTIR spectrum of post-modification cattle tail plant material.
The data obtained after studying the adsorption kinetics of heavy metal ions at different contact times by plant materials modified by H2SO4 were applied to the pseudo-first and pseudo-second types. The adsorption capacity (qe) calculated from false first-type kinetics for Zn (II) and Cd adsorption with the cattle tail material modified with H2SO4 was very close to the experimental data as was the absorptance capacity calculated with the second-type kinetic (qe) [20]. So the modified cattle tail and the adsorption have both false first-degree kinetic and false second-grade kinetic compatibility for Pb and Ni (Table 1).
Table 1. Kinetic results of Zn(II), Cd adsorption in cutting system using modified cattle tail plant material
|
Adsorban type |
Cattle tail sample |
||
| Ağır metal ions | Zn (II) | Cd | |
|
Lying-First Type Kinetic |
qe (mg/g) | 3.453 | 5.723 |
| k1 (min-1) | 0.040 | 0.035 | |
| Δqe (%) | 0.055 | 0.31 | |
| R2 | 0.992 | 0.994 | |
|
Lying-Second Type Kinetic |
qe (mg/g) | 2.321 | 5.844 |
| k1 (min-1) | 0.1357 | 0.027 | |
| Δqe (%) | 0.825 | 1.42 | |
| R2 | 0.996 | 0.998 | |
Correlation coefficients (R2) (Table 2) show that the adsorption data for Zn (II) and Cd are more consistent with the Langmuir isotherm. However, Langmuir acknowledges that the isothermal particle surface is homogeneous and its adsorption potential is stable. In contrast, Freundlich’s isoters are based on the assumption that the surface of the particle is heterogeneous [21]. In industrial applications, wastewater and adsorbents appear to be very well suited to particle surface calculations. The well-adapted Langmuir isotherm (all R2 values > 0.49) suggests that it may also be possible to use this isotherm in industrial applications. The temperature increases and the kinetic energies of the metal ions and adsorbent particles will increase, so that the collision frequency between the absorbance and the metallic ions will be increased, and the attachment of the metals to the surface will also increase [22].
Table 2. Langmuir and Freundlich isotherm parameters were obtained for the adsorption of Ni (II) and Pb(II) in the cutting system using cattle tail (V.cheiranthifolium) plant material.
|
Adsorban |
Cattle tail sample |
||
| Heavy Metal | Zn (II) | Cd | |
| T(°C) | 20 | 20 | |
|
Langmuir Isotherm |
b(mg/g) | 0.634 | 0.178 |
| K (L/mg) | -0.876 | -0.541 | |
| R2 | 0.915 | 0.748 | |
|
Freundlich Isotherm |
Kf | 3.527 | 11.27 |
| 1/n | -1.251 | -1.9265 | |
| R2 | 0.9205 | 0.7838 | |
Effect of the solution’s pH
The effect of pH on the decomposition of Zn (II) and Cd metals at 25°C with a plant material adsorbent of bovine tail (V.cheiranthifolium) is shown in Figure 5. All chemical reactions are controlled by pH, and ions dissolved in a solution at a certain degree of pH willingly form certain compounds. Since adsorption is a balanced event, the required metal ions in the solution will decrease from the moment the solution is in contact with the adsorbent at a suitable pH to the moment it reaches the balance, and after the balance is established, the skin of the solution would be expected to remain unchanged. Many researchers; [23,24,25] believe that the pH in the adsorption case, the surface load of the adsorbent in a liquid solution, the properties of metal ions, the degree of ionization, and the chemical effectiveness of the functional groups on the surface affect ion exchange and complexion.
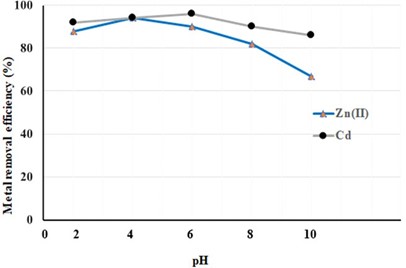
Figure 5. Effect of pH on adsorption capacity in heavy metal waste using cattle tail plant material.
Similar studies have been conducted on the effect of pH on Zn (II) and Cd elimination [26]. pointed out that at low pH levels, the protonation of amino regions on the surface of the cattle tail plant caused a decrease in the chelation capacity of the cattle Tail plant, while the formation of Zn(OH)2 complexes in the basal pH ranges inhibited the adsorption of Zn (II) [27]. emphasized that at high pH 6 values, both ion exchange and the formation of metal hydroxide in aqueous solution can become important mechanisms in the metal removal process
The adsorption effect in Cd removal is above pH 3 and the highest yield in the Cd emission is increased in the pH range 2-5 and decreased after pH 5 [28] in their study of Cd adsorption, achieved the highest rate of consistent increase between pH 2-4 and pH 4, similar to our study. At higher pH levels, the reduced yield may be due to soluble hydroxyl complexes. Argun and ark.[29] explained that Cd(OH)2 is above pH 6 for Cd below pH 8 and that the divalent Cd adsorbent binds to the active surfaces with the O2 ions and causes the pH to fall with the H+ ions it releases into the solution.
Adsorption Balance
Effect of contact time
Modified heavy metal ions were performed with contact times ranging from 0 to 180 minutes to determine adsorption kinetics by cattle tail plant material. The absorption increased rapidly and the balance was reached in between 60 and 180 minutes (Figure 6). After the equilibrium phase, the adsorption yield dropped by almost 1.0%. This suggests that the material field is saturated with heavy metal ions, and then the adsorption stage and the desorption stage can be accompanied [30] argued that chemical adsorption is more prevalent if it takes less than 3 hours to reach the equilibrium; that physical adsorption can be accompanied by 3 to 24 hours; and that diffusion processes can be more effective if they take more than 24 hours. The study that we have done on these statements shows that physical and chemical processes are predominant (equilibrium time is between 60-180 minutes).
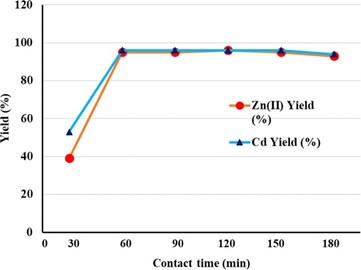
Figure 6. The adsorption capacity of the contact time of the adsorptive capacity in heavy metal waste using cattle tail plant material.
The effect of temperature
Another factor that affects the adsorption process is temperature. The adsorption process can be an endothermic process as well as an exothermic process. A sufficient low-temperature range in physical adsorption caused by Van der Waals forces often leads to a decrease in absorption yield along with rising temperatures. In the chemical adsorption process, which consists of adsorbing the adsorbent ions to the surface by chemical bonding, the absorption yield can be increased or decreased with increased temperature depending on whether the process is endothermic or exothermic and the activation energy. However, it is known that chemical adsorption, which usually occurs at higher temperature ranges compared to physical adsorption, also increases absorption yields along with increased temperature. Thus, in the process of adsorption, temperature is a very important parameter, not only in terms of efficiency, but also in characterizing the type of adsorption [31].
At lower temperatures there is no significant effect on the adsorption capacity, but at higher temperatures. This increase may be due to an increase in the kinetic energy and surface activity of cattle tail plant material [32]. Especially at temperatures of 35 and 40°C, the increase in adsorption capacity is observed to be less than 25 and 30°C (Figure 7). The adsorption capacity values (% yield) have been observed to increase with temperature until a certain balance is reached [33].
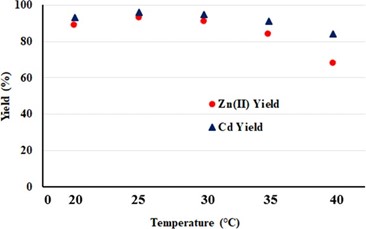
Figure 7. Temperature-dependent change of adsorption capacity in heavy metal waste using cattle tail plant material.
Conclusions
The cattle feed (Verbascum cheiranthifolium) used in our study appears to be an important feature in its use in adsorption studies because of the fact that the plant is not expensive. It also increases the chemical oxygen requirement of the recipient environment, even to a slight extent, due to certain organic substances (such as phenolic substances) that are soluble in the water that the cattle plant contains [34]. The modifications also reduced phenolic compounds, which dissolved in water compared to the raw material, increasing the chemical oxygen requirement. The decrease in the color of the raw material in contact with water has facilitated the analysis of spectroscopic photometric tests. These changes have also been observed in the FTIR analysis. The yields varied with the concentration of heavy metals, but modified cattle feed was found to be around 96% for plant material.
Adsorption isotherm studies have shown that the system is generally more adapted to the Langmuir isotherm. It has been observed that discharge processes can occur spontaneously in thermodynamic terms at normal temperatures, and often as the temperature increases, discharges decrease. It is a notable advantage that all the materials used are not natural and expensive. The modifications are thought to be preferable to classical adsorbents, although the cost increases slightly. Similarly, it is believed that the materials that we have chemically modified in this application study can be used for the adsorption of heavy metals from wastewater.
In this study, we found that adsorption processes can take place autonomously thermodynamically at normal temperatures, and usually with a reduction in temperature, so that there is no need for heating during the process. The natural availability and economics of existing adsorbents is an important advantage. The modifications do not show significant cost increases and are likely to compete with classical adsorbents.
References
- Han, , Qiu, L., Ren, Z., Qiu, Q., 2018, Ceramic Membrane Coupling Process for Advanced Treatment of Electroplating Wastewater, IOP Conf. Ser.: Mater. Sci. Eng. 392, 022037.
- Rajoria, S., Vashishtha, M., Sangal, V.K., 2022, Treatment of electroplating industry wastewater: a review on the various techniques, Sci. Pollut. Res. 29, 72196-72246.
- Li, S., Dai, M., Wu, Y., Fu, H., Hou, X., Peng, C., Lou, H., 2022, Resource utilization of electroplating wastewater: obstacles and solutions, Sci. Water Res. Technol. 8, 484-509.
- Kuppusamy, , Venkateswarlu, K., Thavamani, P., Lee, Y. B., Naidu, R. ve Megharaj, M., 2017, Quercus robur acorn peel as a novel coagulating adsorbent for cationic dye removal from aquatic ecosystems, Ecological Engineering, 101, 3-8.
- Kuppusamy, , Thavamani, P., Megharaj, M., Venkateswarlu, K., Lee, Y. B. ve Naidu, R., 2016, Oak (Quercus robur) Acorn Peel as a Low-Cost Adsorbent for Hexavalent Chromium Removal from Aquatic Ecosystems and Industrial Effiuents, Water, Air, & Soil Pollution, 227 (2), 62.
- Zhao, , Li, Q., Tang, Z., Yang, Y., Wu, J., Gao, P., Qian, Y., Chen, J., Xue, G., 2024, Simultaneous removal of Cr, Cu, Zn, and Cd by nano zero-valent iron modified sludge biochar in high salinity wastewater, Separation and Purification Technology.
- Xia, , Zhang, Y., Chen, Q., Liu, R., Wang, H., 2023, Unraveling adsorption characteristics and removal mechanism of novel Zn/Fe-bimetal-loaded and starch-coated corn cobs biochar for Pb(II) and Cd(II) in wastewater, Journal of Molecular Liquids, Volume 391, Part B.
- Jafaripour, , Rowson, N. A. ve Ghataora, G. S., 2015, Utilisation of residue gas sludge (BOS sludge) for removal of heavy metals from acid mine drainage (AMD), International Journal of Mineral Processing, 144, 90-96.
- Shrestha, , Ban, S., Devkota, S., Sharma, S., Joshi, R., Tiwari, A.P., Kim, H.Y., Joshi, M.K., 2021, Technological trends in heavy metals removal from industrial wastewater: A review, J. Environ. Chem. Eng. 9, 105688. https://doi.org/10.1016/j. jece.2021.105688.
- Qasem, A.A., Mohammed, R.H., Lawal, D.U., 2021, Removal of heavy metal ions from wastewater: a comprehensive and critical review, NPJ Clean Water. 4, 36.
- Wu, C.H., 2007, Adsorption of reactive dye onto carbon nanotubes: Equilibrium, kinetics and thermodynamics, Journal of Hazardous Materials, 144 (1), 93-100.
- Cheremisinoff P.N., Ellerbusch F., 1978, Carbon Adsorption Handbook, Ann Arbor Science Publishers, Inc., New
- Lee, ve Park, T. G., 2011, Facile fabrication of branched gold nanoparticles by reductive hydroxyphenol derivatives, Langmuir, 27 (6), 2965-2971.
- Ho, S., Wase, D.A.J., Forster, C.F. 1996 Kinetic studies of competitive heavy metal adsorption by sphagnum moss peat. Environ. Technol. 17: 71-77.
- Yang, , Xu, Y., Huang, Q., Sun, Y., Liang, X., Wang, L., Qin, X., Zhao, L., 2021, Adsorption characteristics and the removal mechanism of two novel Fe-Zn composite modified biochar for Cd(II) in water, Bioresource. Technol. 333, 125078,
- Wang, , Chen, Q., Liu, R., Zhang, Y., Zhang, Y., 2022, Synthesis and application of starchstablized Fe-Mn/biochar composites for the removal of lead from water and soil, Chemosphere 305, 135494.
- Kuppusamy, , Thavamani, P., Megharaj, M., Venkateswarlu, K., Lee, Y. B. ve Naidu, R., 2016, Oak (Quercus robur) Acorn Peel as a Low-Cost Adsorbent for Hexavalent Chromium Removal from Aquatic Ecosystems and Industrial Effiuents, Water, Air, & Soil Pollution, 227 (2), 62.
- Eltaweil, A.S., Hashem, O.A., Abdel-Hamid, H., Abd El-Monaem, E.M., Ayoup, M.S., 2022, Synthesis of a new magnetic sulfacetamide-ethyl acetoacetate hydrazone-chitosan schiff-base for Cr(VI) removal, J. of Biol. Macromol. 222, 1465–1475
- Shi, , Zhang, H., Shahab, A., Zeng, H., Zeng, H., Bacha, A.U.R., Nabi, I., Siddique, J., Ullah, H., 2021, Efficient performance of magnesium oxide loaded biochar for the significant removal of Pb2+ and Cd2+ from aqueous solution, Ecotox. Environ. Safe. 221, 112426.
- Park, D., Yun, Y.S. ve Park, J. M., 2010, The past, present, and future trends of biosorption, Biotechnology, and Bioprocess Engineering, 15 (1), 86-102.
- Shukla, R., Pai, R.S. 2005 Adsorption of Cu(II), Ni(II) and Zn(II) on modified jüte fibres. Bioresource Technol. 96: 1430–1438.
- Ji, , Zheng, N., An, Q., Sun, S., Wang, S., Li, X., Li, P., Hua, X., Dong, D., Zhao, C., Li, Z., Zhang, W.,2022, The effect of carbonization temperature on the capacity and mechanisms of Cd(II)-Pb(II) mix-ions adsorption by wood ear mushroom sticks derived biochar, Ecotox. Environ. Safe. 239, 113646.
- Deng, R., Huang, D., Zeng, G., Wan, J., Xue, W., Wen, X., Liu, X., Chen, S., Li, J., Liu, C., Zhang, Q., 2019, Decontamination of lead and tetracycline from aqueous solution by a promising carbonaceous nanocomposite: Interaction and mechanisms insight, Technol., 283, 277-285, 10.1016/j.biortech.2019.03.086.
- Abdić, Š., Memić, M., Šabanović, E., Sulejmanović, J. ve Begić, S., 2018, Adsorptive removal of eight heavy metals from aqueous solution by unmodified and modified agricultural waste: tangerine peel, International Journal of Environmental Science and Technology, 15 (12), 2511-2518.
- Afroze, ve Sen, T. K., 2018, A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents, Water, Air, and Soil Pollution, 229 (7).
- Karthikeyan, , Anbalagan, K. ve Andal, N. M., 2004, Adsorption dynamics and equilibrium studies of Zn (II) onto chitosan, Journal of Chemical Sciences, 116 (2), 119-127.
- Anirudhan, T. S. ve Suchithra, P. S., 2008, Synthesis and characterization of tannin immobilized hydrotalcite as a potential adsorbent of heavy metal ions in effiuent treatments, Applied Clay Science, 42 (1-2), 214-223.
- Ashwini, A., Udayasimha, L., Vyshnavi, D. R. ve Usha, H. S., 2018, Comparative Study On Removal Of Copper From Aqueous Solution By Modified And Non Modified Sawdust Adsorption, International Journal of Pure and Applied Mathematics, 120 (6), 6709-6725.
- Argun, M. E., Dursun, S., Ozdemir, C. ve Karatas, M., 2007, Heavy metal adsorption by modified oak sawdust: thermodynamics and kinetics, J Hazard Mater, 141 (1), 77-85.
- Ho, S., Nag, J.C.Y., McKay G. 2000 Kinetics of pollutant sorption by biosorbents: review. Separation and Purification Methods 29(2): 189–232.
- Kothavale, P., Sharma, A., Dhavale, R.P., Chavan, V.D., Shingte, S.R., Selyshchev, O., Dongale, T.D., Park, H.H., Zahn, D.R.T., Salvan, G., 2022, Hyperbranched amino modified magnetic nanoparticles for simultaneous removal of heavy metal ions from aqueous solutions Mater. Chem. Phys., 292, Article 126792.
- Singh, K., Srivastava, B., 1999, Removal of basic dyes from aqueous solutions by chemically treated Psidium Guyava leaves, Indian J Environ Health, 41, 333.
- Yu, , Hu, J., Yu, Y., Ma, D., Gong, W., Qiu, H., Hu, Z., Gao, H.W., 2021, Facile preparation of sulfonated biochar for highly efficient removal of toxic Pb(II) and Cd(II) from wastewater, Sci. Total. Environ. 750, 141545
- Gürü, , Venedik, D., and Murathan, A., 2008, Removal of trivalent chromium from water using low-cost natural diatomite, J. Hazardous Materials, 160, 318-323.
This article licensed under the Creative Commons Attribution 4.0 International License CC-BY 4.0., which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are properly credited.
