NL Journal of Agriculture and Biotechnology
(ISSN: 3048-9679)
Chemical Amount of Chamomile Populations in Albania
Author(s) : Alma Imeri, Ndricim Zhuri. DOI : 10.71168/NAB.01.01.103
Abstract
Matricaria chamomilla L. is one of the most important medicinal plants in Albania. It has been used for centuries and its usage recently still has great importance. Beside its traditional application forms it is also used in the homeopathy, in several cosmetics; nowadays is an increasing demand on chamomile. Beside its importance as a medicinal plant, chamomile has great economic significance as well, because the dried chamomile flowers are one of the most important exported products. In Albania there is not yet a tradition of cultivation of Matricaria chamomilla L. It is a wild plant spread and its abundance due to climate is almost found in all Albania. My survey is related to the amount of some component in essential oil of Matricaria chamomilla L, cultivated and wild growing. Based on our results giving advices for the improvement of technical knowledge which can increase the effectiveness of the selection work carried out on chamomile.
Introduction
German chamomile, Matricaria chamomilla L. (syn. Chamomilla recutita (L.) Rauschert), which belongs to the Asteraceae family, is a very important medicinal plant species [4; 5]. Chamomile is a native plant of South and East Europe. However, at present it has spread over nearly all the European continent. The plants can be found in North Africa, Asia, North and South America, Australia and New Zealand [4]. The flowers of German chamomile accumulate blue essential oil from 0.2 to 1.9% [6]. As a medicinal plant, its dried flowers are an old age remedy, known in ancient Egypt, Greece and Rome [3]. Nowadays, in phytotherapy, flower anthodia are mainly used. Pharmacological properties include anti- inflammatory, antiseptic, carminative, healing, sedative and spasmolytic activity [5]. According to Yanive and Palevitch (1982) and Bernath (1986) [1], essential oil content and composition of essential oil in plants varies and is due to the genetic and Environmental factors. Well irrigated chamomile plants produced flowers with high content of essential oil [7] and flower yield [2] Therefore, the main objective of the present study was to measure the essential oil content and composition of German chamomile based on best practice of cultivation.
The biological and therapeutical application of plants of the compositae with nearly 120 genera and more than 15,000 species in Albania is more the result of systematically conducted chemical and pharmacological research than of tradition. In addition, the compositae has attracted many chemists and biochemists and substantial research has been built up over the past four decades on the chemical constituents of individual species and genera. A study of the early herbals reveals that a surprisingly large number of plants of the compositae were used for their curative properties. Undoubtedly, the wide medicinal use of many compositae inspired the organic chemists at the turn of the century to explore the chemistry in order to identify the active constituents. Compositae in fact are exceptionally rich, both in the range of secondary compounds present and also in the number of complex structures known of any one class [8].
Many other substances elaborated by the family are toxic or show other significant physiological activity and this may be one reason why plants of the compositae are rarely used in human diets or for animal fodder. Several classes of plant compound are characteristic of this family notably the terpenoid - based sesquiterpene lactones, the fatty acid derived polyacetylenes and the polysaccharide fructans. Many structures discovered for first time in this family have served as models for the synthesis of biologically active compounds and have promoted research into the activity of analogous structures. New screening methods and isolation techniques have made it possible to elucidate the mode of action of old drugs and thereby reintroduce them into modern therapy [8]. The highest content of essential oil and azulenic substances was found in normally ripe flower heads [9]. The essential oil content of chamomile flowers was 0.24 - 1.9% [8].
For a long time, the only known active principle in the chamomile oil was azulene. The name azulene was given to the parent compound of the azulene series C10H8 [10]. Azulene from M. chamomilla L. has been named chamazulene (camazulene) in order to differentiate it from the azulenes contained in other essential oils [11]. One of the most striking properties of the azulenes is their intense blue or blue-violet colour, noticeable even at very high dilution. Azulenes are decomposed by permanganate, even at low temperature, to small fragments and therefore there is no six membered aromatic ring in azulenes [10]. Chamazulene (l,4-dimethyl-7-ethylazulene) is of intensive characteristic blue colour owing to its conjugation system of five double bonds. Chamazulene is a bicyclic hydrocarbon [12].
Chamazulene, one of the major component of the oil, has pain-relieving, wound healing, antispasmodic, antiinflammatory antimicrobial properties [13]. Antiphilogistic activity of prochamazulene has been determined and found to be at least equal to that of chamazulene [14]. Chamazulene when tested on dextran- induced rat paw oedema showed the highest antiinflammatory activity.[15] Chamazulene show only slight activity and ineffective against tetanus toxin [16]. Chamazulene is a special histamine-releasing substance. Chamazulene or the blue oil o f chamomile does not have an antiinflammatory effect, but it enhances sluggish inflammatory reaction and make them more intensive [17].
Bisabolol, another constituent of the oil, has antiinflammatory, antimicrobial and antipeptic activities [13]. Laevorotatory form which is found in chamomile has more effective antiphlogistic and spasmolytic effect then the racemate (dextrarotatory form). The chemistry of bisabolol has been fruitfully extended by the partial synthesis of bisabolol ethers and esters. Most of these synthetic compounds are more active and usually have lower toxicity than natural a-bisabolol [8]. Bisabolol exhibited varying degrees of fungistatic activity. It has significant effects at only 100 mg/ml and is fungicidal to Candida albicans following a 30 minutes’ exposure of the yeast to a 1000 mg/ml concentration [18].
Material and Methods
Steam Distillation
Chamomile essential oil was isolated by steam distillation (READ 1992). Hydro-distillation lasted for 2 hours into n-hexane; sample weights were 2g of drug dry matter. Samples have been grown in wild and cultivated. They are German chamomile well studied and certified by genetic bank near department of agronomy science in Agricultural University of Tirana. Plants have been cultivated in south of Albania (Korça district) and in northern part of Albania (Lezha district).
A modified distillation apparatus by Coocking & Middleton was used (HUMPHREY 1992). Gas chromatography (GC)
The compounds of essential oil were determined by means of Hewlett-Packard 5890 Series II system, with capillary column HP-5, FID detector, split-splitless system for injection and automatic injector HP 7673. The operating conditions were: injection temperature 150°C, detector temperature 250°C, carrier gas nitrogen. Sample sizes 1.0 μl were used and manual type of injection. The composition of chamomile essential oil was determined by capillary GC analysis: Hewlett- Packard 5890 Series II with FID and split-splitless system for injection. The column HP-5 (50 m long × 0.20 mm i. d.) was used. The following temperature program was used: 90oC (0 min), then 10oC/min to 150oC (5 min), 5oC/min to 180oC (3 min), 7oC/min to finally isothermal 280oC for 25 min; nitrogen was used as carrier gas. Detector temperature 250°C, carrier gas nitrogen (flow velocity 274 mm/s), auxiliary gases were nitrogen (30 ml/min), hydrogen (30 ml/min), air (400 ml/min). Peak areas and retention times were measured by electronic integration with a Hewlett-Packard 3396 Series II integrator.
Results and Discussions
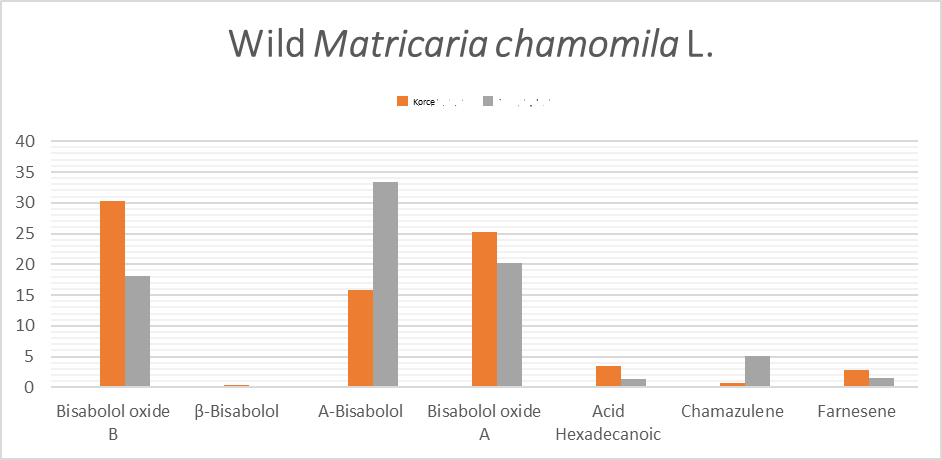
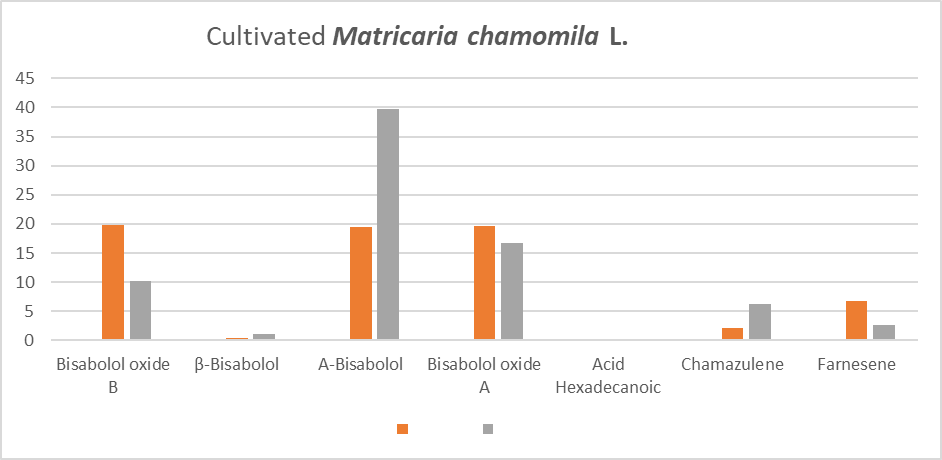
There are a lot of components presents in the essential oils of Matricaria chamomilla L. The focus of this study is related to the amount of some component (used in the industry, pharmacology, home therapy), in essential oil of Matricaria chamomilla L. Using cultivated plants and wild growing, the amount of essences is different and should be discussed. Based on our results Bisabolol has high amount in cultivated plants in Lezha, so is recomented to be used and cultivated more. This cultivated plants in same place have high amount of Chamazulene well known for antifungal effect.
| Compound name | Formula | Korce Wild | Korce Cultivated | Lezhe Wild | Lezhe Cultivated | |
| 1. | Farnesene | C15H24 | 2.85 | 6.82 | 1.47 | 2.62 |
| 2. | Bisabolol oxide B | C15H26O2 | 30.36 | 19.78 | 18.11 | 10.24 |
| 3. | Bisabolone oxide A | C15H24O2 | 25.25 | 19.64 | 20.29 | 16.78 |
| 4. | ß-Bisabolol | C15H26O | 0.36 | 0.48 | 0.17 | 1.01 |
| 5. | a -Bisabolol | C15H26O | 15.81 | 19.45 | 33.35 | 39.69 |
| 6. | Chamazulene | C14H16 | 0.66 | 2.1 | 5.06 | 6.61 |
| 7. | Acid hexadecanoic | C16H32O2 | 3.45 | 1.41 |
Recommendation
As is shown to the graf 1 and garf 2 the amount of bisabolol is varied to wild cultivars and cultivated. We recommend the wild practices for this culture to be grown and collected using the best practices for short term export of this culture. In the recent past the aim of essential oils have alternately shifted from culinary use to pharmaceutical and therapeutic use. So focused on the amount of these essences is essential for the industry and science as well. Finally, composition of the essential oils could be affected by the geographical environment, geomorphological state of the plants grown, physical and chemical characteristics of soil, plant age, better oil extraction method.
SHEQERAS/KORCE: Popullate Natyrore
| CHAMOMILLA RECUTITA | CHAMOMILE | BLUE | 11 | |
| COMPOUND | RT | KOVATS | AREA | %AREA |
| trans-2-hexanal | 9.35 | 853 | 867 | 0.9% |
| tricyclene | 9.55 | 884 | 1412 | 1.46% |
| α-pinene | 13.23 | 931 | 943 | 0.97% |
| camphene | 14.09 | 940 | 199 | 0.21% |
| sabinene | 15.67 | 971 | 194 | 0.2% |
| β-pinene | 16.36 | 983 | 151 | 0.16% |
| β-myrcene | 16.74 | 987 | 1019 | 1.05% |
| γ-muurolene | 18.74 | 1509 | 167 | 0.17% |
| α-farnesene | 19.73 | 1511 | 6599 | 6.82% |
| α-muurolene | 20.94 | 1519 | 285 | 0.29% |
| α-bulnesene | 20.98 | 1523 | 486 | 0.5% |
| γ-cadinene | 21.17 | 1542 | 360 | 0.37% |
| calamenene | 22.37 | 1546 | 914 | 0.94% |
| α-acoradiene | 24.26 | 1551 | 142 | 0.15% |
| cadina-1,4-diene | 25.04 | 1557 | 871 | 0.9% |
| δ-cadinene | 25.71 | 1567 | 345 | 0.36% |
| tremetone | 26.27 | 1613 | 1165 | 1.2% |
| caryophyllene oxide | 27.03 | 1614 | 216 | 0.22% |
| viridiflorene | 27.56 | 1623 | 2046 | 2.11% |
| dillapiole | 28.32 | 1641 | 347 | 0.36% |
| cubebol | 29.57 | 1653 | 347 | 0.36% |
| β-bisabolole | 29.99 | 1659 | 463 | 0.48% |
| τ-muurolol | 30.84 | 1663 | 1223 | 1.26% |
| α-bisabololoxide B | 31.3 | 1675 | 19140 | 19.78% |
| bulnesol | 32.33 | 1681 | 3543 | 3.66% |
| α-bisabolole | 33.85 | 1709 | 18816 | 19.45% |
| chamazulene | 37.99 | 1771 | 2036 | 2.1% |
| α-bisabololoxide A | 45.43 | 1780 | 19000 | 19.64% |
| cis-en-in-dicycloether | 49.98 | 1923 | 12696 | 13.12% |
| trans-en-in-dicycloether | 50.12 | 1958 | 752 | 0.78% |
| total | 96744 | 100 |
SHEQERAS/KORCE: Popullate natyrore
| CHAMOMILLA RECUTITA | CHAMOMILE | BLUE | 6 | |
| COMPOUND | RT | KOVATS | AREA | %AREA |
| neryl acetate | 26.33 | 1371 | 88 | 0.01% |
| α-copaene | 26.92 | 1395 | 395 | 0.06% |
| decanone | 27.31 | 1406 | 1950 | 0.28% |
| decanonic acid | 28.47 | 1413 | 289 | 0.04% |
| γ-muurolene | 30.04 | 1509 | 175 | 0.02% |
| α-farnesene | 30.33 | 1514 | 20142 | 2.85% |
| α-muurolene | 30.82 | 1520 | 1331 | 0.19% |
| α-bulnesene | 31.01 | 1526 | 2278 | 0.32% |
| γ-cadinene | 31.73 | 1540 | 899 | 0.13% |
| calamenene | 32.77 | 1545 | 664 | 0.09% |
| α-calacorene | 33.61 | 1568 | 299 | 0.04% |
| trans-nerolidol | 33.81 | 1571 | 336 | 0.05% |
| epiglobulol | 34.01 | 1585 | 714 | 0.1% |
| junenol | 35.01 | 1591 | 1560 | 0.22% |
| spatulenol | 35.65 | 1602 | 5981 | 0.85% |
| β-eudesnol | 36.33 | 1606 | 5826 | 0.83% |
| globulol | 36.97 | 1610 | 17411 | 2.47% |
| tremetone | 37.74 | 1612 | 2422 | 0.34% |
| caryophyllene oxide | 38.23 | 1615 | 693 | 0.1% |
| viridiflorene | 39.08 | 1625 | 2356 | 0.33% |
| dillapiole | 39.58 | 1642 | 3768 | 0.53% |
| cubebol | 40.41 | 1655 | 1878 | 0.27% |
| β-bisabolole | 40.89 | 1660 | 2534 | 0.36% |
| τ-muurolol | 41.8 | 1668 | 9583 | 1.36% |
| α-bisabololoxide B | 42.41 | 1678 | 214240 | 30.36% |
| bulnesol | 43.01 | 1683 | 900 | 0.13% |
| valerianol | 43.37 | 1687 | 3371 | 0.48% |
| cadalene | 44.5 | 1705 | 620 | 0.09% |
| α-bisabolole | 44.9 | 1710 | 111557 | 15.81% |
| chamazulene | 55.81 | 1772 | 4657 | 0.66% |
| α-bisabololoxide A | 57.65 | 1782 | 178174 | 25.25% |
| cis-en-in-dicycloether | 59.25 | 1928 | 83188 | 11.79% |
| trans-en-in-dicycloether | 59.60 | 1940 | 984 | 0.14% |
| hexadecanoic acid | 60.3 | 1965 | 24340 | 3.45% |
| Total | 705603 | 100 |
MAB / LEZHE : Popullate natyrore
| CHAMOMILLA RECUTITA | CHAMOMILE | BLUE | 13 | |
| COMPOUND | RT | KOVATS | AREA | %AREA |
| trans-2-hexanal | 9.19 | 846 | 3063 | 0.67% |
| α-pinene | 13.59 | 927 | 927 | 0.2% |
| β-pinene | 16.05 | 979 | 311 | 0.07% |
| β-myrcene | 16.43 | 983 | 973 | 0.21% |
| trans-β-fernesen | 18.64 | 1461 | 346 | 0.08% |
| α-farnesene | 19.43 | 1512 | 6756 | 1.47% |
| α-muurolene | 20.11 | 1523 | 2575 | 0.56% |
| α-bulnesene | 20.73 | 1525 | 1425 | 0.31% |
| γ-cadinene | 23.18 | 1529 | 155 | 0.03% |
| cadina-1,4-diene | 24.79 | 1554 | 1884 | 0.41% |
| δ-cadinene | 24.89 | 1560 | 676 | 0.15% |
| α-amorphen | 25.37 | 1561 | 646 | 0.14% |
| α-calacorene | 25.93 | 1564 | 966 | 0.21% |
| epiglobulol | 26.85 | 1582 | 545 | 0.12% |
| spatulenol | 27.24 | 1603 | 1297 | 0.28% |
| caryophyllene oxide | 28.36 | 1612 | 1080 | 0.23% |
| cubebol | 29.22 | 1651 | 959 | 0.21% |
| β-bisabolole | 29.65 | 1659 | 793 | 0.17% |
| τ-muurolol | 30.45 | 1666 | 8350 | 1.82% |
| α-bisabololoxide B | 30.94 | 1672 | 83312 | 18.11% |
| bulnesol | 31.92 | 1681 | 4534 | 0.99% |
| α-bisabolole | 33.54 | 1718 | 153365 | 33.35% |
| chamazulene | 43.33 | 1773 | 23276 | 5.06% |
| α-bisabololoxide A | 44.89 | 1778 | 93316 | 20.29% |
| cis-en-in-dicycloether | 49.54 | 1922 | 61576 | 13.39% |
| trans-en-in-dicycloether | 49.68 | 1937 | 296 | 0.06% |
| hexadecanoic acid | 51.23 | 1962 | 6507 | 1.41% |
| Total | 459909 | 100 |
MAB / LEZHE : Popullate e kultivuar
| CHAMOMILLA RECUTITA | CHAMOMILE | BLUE | 5 | |
| COMPOUND | RT | KOVATS | AREA | %AREA |
| trans-β-fernesen | 18.56 | 1465 | 604 | 0.1% |
| α-farnesene | 19.34 | 1517 | 16447 | 2.62% |
| α-bulnesene | 20.45 | 1523 | 368 | 0.06% |
| γ-cadinene | 20.61 | 1541 | 1396 | 0.22% |
| calamenene | 20.78 | 1546 | 152 | 0.02% |
| epiglobulol | 23.89 | 1584 | 972 | 0.15% |
| junenol | 24.68 | 1596 | 4168 | 0.66% |
| spatulenol | 25.24 | 1599 | 493 | 0.08% |
| β-eudesnol | 25.83 | 1603 | 1329 | 0.21% |
| globulol | 26.53 | 1605 | 695 | 0.11% |
| tremetone | 27.13 | 1609 | 2133 | 0.34% |
| caryophyllene oxide | 27.81 | 1611 | 403 | 0.06% |
| viridiflorene | 28.2 | 1623 | 477 | 0.08% |
| dillapiole | 29.06 | 1640 | 1171 | 0.19% |
| cubebol | 29.48 | 1651 | 637 | 0.1% |
| β-bisabolole | 30.26 | 1659 | 6331 | 1.01% |
| τ-muurolol | 30.27 | 1664 | 6239 | 0.99% |
| α-bisabololoxide B | 30.72 | 1679 | 64329 | 10.24% |
| valerianol | 31.38 | 1688 | 327 | 0.05% |
| α-bisaboloneoxide A | 31.69 | 1703 | 6811 | 1.08% |
| cadalene | 32.69 | 1706 | 391 | 0.06% |
| α-bisabolole | 33.32 | 1709 | 249225 | 39.69% |
| chamazulene | 42.82 | 1770 | 38705 | 6.16% |
| α-bisabololoxide A | 44.36 | 1780 | 105352 | 16.78% |
| (ζ,ε)-farnesyl acetate | 45.91 | 1843 | 11982 | 1.91% |
| cis-en-in-dicycloether | 49.12 | 1927 | 107862 | 17.18% |
| trans-en-in-dicycloether | 50.12 | 1939 | 406 | 0.068 |
| Total | 629405 | 100 |
Conclusion
My survey is related to the amount of some component in essential oil of Matricaria chamomilla L, cultivated and wild growing. Based on our results giving advices for the improvement of technical knowledge which can increase the effectiveness of the selection work carried out on chamomile.
References
- Bernath, J., 1986. Production Ecology of Secondary Plant Products. In: Herb Spice and Medicinal Plants Recent Advances in Botany Horticulture and Pharmacology, Craker, L.E. and J.E. Simon (Eds.). Oryx Press, Phoenix, Arizona, pp : 185-234.
- Hornok, , 1992. Cultivation and Processing of Medicinal Plants. Budapest, Academic, Hungary, pp: 246-254.
- Isaac, O., 1989. Recent Progress in Chamomile Research-medicines of Plant Origin in Modern Therapy. Prague, Czecoslovakia, pp: 7.
- Ivens, M., 1979. Stinking mayweed. New Zealand J. Agric., 18: 21-22.
- Salamon, , 1992. Chamomile: A medicinal plant. Herb Spice Med. Plant Dig., 10: 1-4.
- Bradley, , 1992. The British Herbal Compendium. British Herbal Medicine Association, London
- Kerekes, , 1962. Effect of water on flower-yield and active substance of chamomile Matricaria chamomilla L. Herba Hungarica, 1: 55-55.
- Heywood, H., Harbome, J. B., and Turner B. L., (1977), The Biology and Chemistry of the Compositae, volume one, Academic press, London. 412-413
- Akacic, B., and Kustrak, D., (1960), Evaluation of flos chamomillae, Farm. Glasnik, 16, 419-423; through Chem. , 55, 16909, (1961).
- Maxwell, Gordon, (1952), The azulene, Rev., 50, 127-200.
- Guenther, , (1952), The Essential Oil, Individual Essential Oils o f the Plant Families, D. Van Nostrand and Company, Inc. London. Volume five, 438 - 445.
- Heeger, f., Bauer, K. H., and Poethke W., (1946), Matricaria chamomilla L.,true chamomile, pharmazie 1, 210-18: through Chem. Abstr. 41, 6021, (1947).
- Leung, A. Y., (1980). Encyclopedia of Common Natural Ingredients used in Food, Drugs and Cosmetics, Wiley - interscience, John Wiley and Sons, New York, 100 -112.
- Cekan, , Herout, V. and Sorm, F., (1954), Chamazulene precursor from chamomile, Chemistry and industry, 604-5; through Chem. Abstr. 49, 497C (1955).
- Verzar Petri; Gizella; Szegi, Jozsef; Marczal Gabriella, (1979), Pharmacological effect of certain chamomile Acta. Pharm. Hung. 49 (1), 13-20; through Chem. Abstr,90 115339t; (1979)
- Mose, J. R., and Luka, G., (1957), Antibacterial action o f some ethereal oils and their components Arzneimitted torsch 7, 687-692; through Chem. Abstr. 52, 5544, (1958).
- Vane, R., Ferreira, S. H., (1979), Anti-inflammatory Drugs, Handbook of Experimental Pharmacology, Sprunger- Verlag Berhn Heidelberg New York, 50/11, 726-729,
- Szalontai Marianne; V erzur-Petri Gizella; Florian, Ede; (1977), Study o f the anti mycotic effects of biologically active components ofMatricaria chamomilla L., Parfum, Kosmet. 58 (5), 121-127; through Chem. Abstr. 87, 96715, (1977).
This article licensed under the Creative Commons Attribution 4.0 International License CC-BY 4.0., which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are properly credited.
