NL Journal of Veterinary and Animal Nutrition
Comparative Analysis of Antibody Detection Against Newcastle Disease Virus in Broilers, Local Chickens and Guinea Fowls in Live Birds Market in Sokoto, Nigeria
Author(s) : Usman Musa, Hauwa’u Umar Mungadi, Muhammad Abubakar Dan Ali, Usman Muhammad Mera, David Ishola Olayinka, Yusha’u Shuaibu Baraya.
Abstract
Newcastle Disease Virus (NDV) constitutes a major constraint to the poultry production system in Nigeria. This study was carried out to investigate the seroprevalence of NDV antibodies in broilers, local chickens and guinea fowls slaughtered in live bird markets (LBMs) in Sokoto metropolis, Nigeria. One hundred and fifty blood samples were collected and screened for antibodies against NDV using Haemagglutination Inhibition (HI). An overall seroprevalence rate of 93.3% was recorded in this study. Seroprevalence based on different species revealed a higher prevalence of 96% in broilers, followed by local chicken and guinea fowls with 92% each. The differences in seroprevalence among the species were not statistically significant (p > 0.05). Thestudy indicated that NDV is endemic in the population and warrants the need for a regular strategic vaccination program against Newcastle disease (ND) in local chickens and guinea fowls in the Sokoto metropolis. Keywords: Broilers, Guinea fowls, Live birds’ markets, Local chickens, Newcastle diseasevirus, Seroprevalence.
Introduction
Newcastle disease (ND), a highly contagious viral disease, affects over 250 species of birds of all ages [1] and is caused by Newcastle disease virus (NDV), a linear, non-segmented, single stranded, enveloped, negative sense RNA virus that belongs to the genus Rubula virus of the sub-family Paramyxovirinae and family Paramyxoviridae [2].
According to [3], NDV has been divided into five different pathotypes based on differences in virulence: viscerotropic velogenic, neurotropic velogenic, mesogenic, lentogenic, and asymptomatic enteric. Mortality from the disease caused by mesogenic strains may reach 25%, whereas that from velogenic strains may reach 100% and ranges from 80-90% in adults [4,5]. The main clinical symptoms of ND are weakness, depression, loss of appetite, thirst, immobility, nasal and ocular discharges, cyanosis of the comb and wattle, greenish watery diarrhea, decreased egg production, weight loss, and mortality.[6] Gross lesions include petechial hemorrhages, ulcers with elevated borders, pneumonic lungs, hemorrhages in the trachea, air sacs, brain, and spleen, among others [6].
Newcastle Disease Virus (NDV) is a significant viral disease of economic importance, rated as one of the biggest obstacles to the meaningful development of rural poultry husbandry, resulting in catastrophic loss to the poultry industry [7,8].
Severe losses from mortality, decreased egg production and lowered feed conversion efficiency occur as a result of exposure to vvND. The lentogenic form is responsible for erosive losses in broilers including lowered gain and feed conversion efficiency and elevated mortality and condemnation. The severity and financial impact depend on climatic and management stress, concurrent exposure to pathogenic Escherichia coli (E. coli) and other viral respiratory disease and immunosuppressive agents. The cost and consequences (respiratory stress) of vaccination are significant especially during winter and following immunosuppression. Disruption of trade and the cost of eradication of Velogenic viscerotropic Newcastle disease (vvND) in non-endemic countries impose a significant burden on producers and the public sector after outbreaks [9].
The detection of NDV antibodies in live birds is crucial for monitoring the disease’s prevalence and controlling its spread. Hemagglutination inhibition technique (HI) is a reliable and cost-effective method for detecting NDV antibodies in serum samples. The results of this study will provide valuable information on the prevalence of NDV antibodies in broilers, local chickens and guinea fowl in live bird markets in Sokoto. This information will help in developing strategies for controlling the spread of NDV in the poultry industry, which will ultimately lead to improved economic outcomes for farmers and the community. Additionally, the study will contribute to the existing knowledge on NDV and its potential transmission to other avian species
The aim of this study was to detect NDV antibodies in broilers, local chickens and guinea fowls in live bird markets in Sokoto using the HI technique. This study was undertaken to determine the prevalence of NDV antibodies in broilers, local chickens and guinea fowls in live bird markets in Sokoto and compare the prevalence of NDV antibodies among different bird species in live bird markets in Sokoto.
Materials and Methods
Study Area
The study was conducted in live bird markets (LBMs) in Sokoto metropolis, the capital of Sokoto State, Nigeria. Geographically, Sokoto state is situated on latitude 12ºN and 13º58N and is 308m above the sea level. Sokoto State occupies an area of short grass savannah vegetation in the South and thorn in the North. It shares boundaries with Zamfara State to the East, Niger Republic to the North and Kebbi State to the West and Southwest [10].
Sokoto metropolis is mainly made of Sokoto North and South Local Government Areas, however, some parts of Dange Shuni, Wamakko, and Kware Local Government Areas constitute part of this metropolis [10].
Experimental birds and sample size
Convenience sampling was used to survey live birds markets, sample size was determined at 14.72% previous prevalence reported by [11] for local chickens, 17% previous prevalence [12] for both guinea fowls and broiler chicken at 95% confidence interval, with desired precision of 5%, using the formula:
N= z2p(1-p)/d2 [13],
Where:
n= simple size,
z= score for given interval which is 1.96 (SE) at 95% confidence interval,
p= prevalence at 14.72% [12] for local chicken and 17% [13] for other species. d2= precision at 0.05 (5%).
For local chicken
N= (1.96)2x0.1472x(1-0.14.72)/(0.05)2 =192.897738344, =193.
For broiler and guinea fowl
N= (1.96)2x0.17x(1-0.17)/(0.05)2 = 3.18416x0.17x0.83/0.0025= 217.
Total minimum number of samples to be collected was 410
Due to circumstance and economic reasons only 50 samples were collected from each species (local chicken, guinea fowls, and broiler which made the total samples to 150.
Sample Sera (collection)
In a convenient sampling technique, 4ml blood samples were collected at slaughter from broilers, local chickens, and guinea fowl brought from different parts of the State to be sold at the largest poultry live bird markets (LBMs) within Sokoto metropolis. A total of 150 samples were collected, Samples were properly labeled and transported in a cool box to the clinical pathology laboratory of the Department of Veterinary Pathology, Usmanu Danfodiyo University, Sokoto for HI test. The samples were kept in a slanting position till clot formation. Sera were harvested, transferred into a sterile serum bottle, and stored at −20°C until required for analyses.
Vaccine (antigen)
Newcastle disease vaccine virus Lasota strain obtained from National Veterinary Research Institute (NVRI) Vom was used as an antigen after reconstitution of 200 dose vial in 2ml of distilled water. The HA titre was determined as baseline.
Haemagglutination (HA) test and haemagglutination inhibition (HI) test
Preparation of washed RBC (Indicator)
Chicken blood was collected into a sterile anticoagulant tube containing Ethylenediaminetetraacetic acid (EDTA), using a sterile hypodermic syringe under sterile conditions. The blood was then centrifuged at 3000 rpm for 10 minutes and the supernatant, with the white blood cell layer, was removed and discarded. The red blood cells were then washed 4 times using Phosphate buffer saline (PBS) with the removal of the supernatant at each wash. After the red blood cells were properly washed, 1% of Chicken RBC was then prepared by adding 99 ml of Phosphate buffer saline (PBS) to 1ml of the washed RBC, the solution was stored in the refrigerator at -4°C till use.
Haemagglutination (4HA UNIT) test
25 µl of PBS was added into all wells and 25 µl of antigen (vaccine) was added into well 1 and 12, then serial dilution was performed from well 1 to well 11, and 25 µl was discarded. 25 µl of 1% Chicken RBC was added into all wells mix and incubated at room temperature for 30 to 45 minutes and took the reading.
Haemagglutination Inhibition (HI) test
Using a Multichannel pipette, 0.025ml (25µl) volume of normal saline was put into each well in the clean microtiter plates. 0.025 ml (25µl) volume of each sera sample was then added to the first column of each row and a 2-fold serial dilution was done with the last volume left in the tips discarded. 0.025 ml (25µl) volume of the reconstituted virus (4HAU antigen) was added to each well on the microtiter plates [12]. The mixture was shaken and incubated at room temperature for 25 minutes. After 25 minutes, 0.025 ml (25µl) of 1% washed chicken RBC is added, and then it was gently mixed and then left for 25 minutes. The result was read and recorded by assessing the highest dilution of serum causing complete inhibition of 4 HA units of antigen [12]. The test was conducted at the veterinary clinical pathology laboratory, UDUS.
Statistical analysis
All data were entered into Microsoft® Excel. 1-way ANOVA was used to measure the strength of the association between species and prevalence of NDV.
Results
The result revealed an overall seroprevalence of 93.3%. Seroprevalence based on different species showed a higher prevalence of 96% in Broilers, followed by local chickens and Guinea fowls with prevalence of 92% each. The difference in seroprevalence among the different Species was not statistically significant P>0.05 (Table 1).
Table 1: Seroprevalence of NDV in three avian species in live birds Market in Sokoto
| SPECIES | TOTAL NO. EXAMINED | POSITIVE | NEGATIVE | PROPORTION% |
| Broilers | 50 | 48 | 2 | 96 |
| L/chicken | 50 | 46 | 4 | 92 |
| G/ fowls | 50 | 46 | 4 | 92 |
| TOTAL | 150 | 140 | 10 | 93.3 |
Table 2: Distribution of Newcastle Disease Antibody titre from broilers, local chickens and guinea fowls in live birds Market Sokoto.
| SPECIES | NO. OF SERA
TESTED |
NO. (%) OF SERA WITH TITRE ≤3 Log2 | NO. (%) OF SERA WITH TITRE ≥4 Log2 |
| Broilers | 50 | 2 (4%) | 48 (96%) |
| Local chickens | 50 | 4 (8%) | 46 (92%) |
| Guinea fowls | 50 | 4 (8%) | 46 (92%) |
Mean HI titre
The mean HI titer for the respective species are; Guinea fowl =6.5, Broilers =7.2 and Local chicken 6.7.
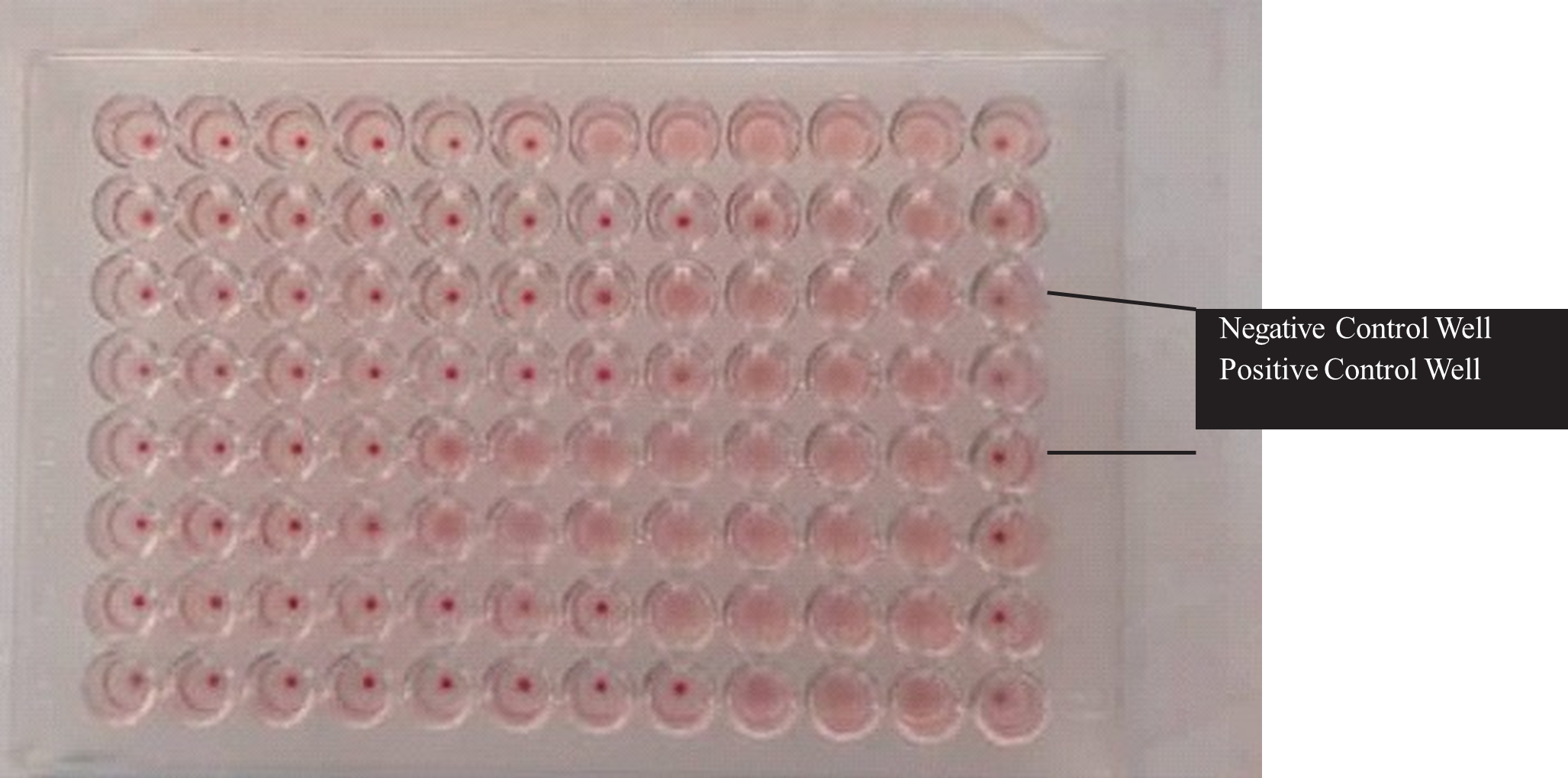
Plate 1: 96 well (U-bottom) plate after HI test
Hemagglutination is seen in some of the wells while absent in some wells.
Statistical Analysis
Statistical analysis revealed no significant differences in the HI test results among the three groups (Broilers, Local chicken, and Guinea fowl) as evidenced by a non-significant F-ratio (F = 0, p = 1) in the Between Groups comparison. The p-value is greater than the conventional significance level of 0.05, suggesting that there is no evidence to reject the null hypothesis.
In summary, based on the HI test results, there are no statistically significant differences in the means of the three groups. This implies that, in the context of the HI test, the performance of Broilers, Local chicken, and Guinea fowl is comparable.
Discussion
Newcastle disease is one of the most economically important infectious diseases of avian species, thus, quick detection and identification of the virus is of importance to effectively control the disease. To confirm the presence of the virus rapidly in the serum, haemagglutination inhibition test were used in this study. This test is commonly used by many laboratories in the world to determine the virus presence and measure the titer of infectivity of the virus from different species.
The present study detected the presence of antibodies against NDV in One hundred and fifty sera samples collected from broilers, local chickens and guinea fowls (50 from each species) in live bird markets in Sokoto metropolis, Sokoto State Nigeria using the HI test. This indicates that Newcastle disease virus infection is endemic in the area, and the markets are serving as mixing point of infected birds with susceptible ones as some of these birds are taken back home to be reared. The sellers and buyers as well as those processing the meat may also be veritable vehicle of transmission and spread of the disease. This is therefore, a great threat to rural and commercial poultry production in Sokoto State. The implication of the spread and the carrier status of the rural household chickens could be of importance considering the fact that free ranged chickens were reported to constitute almost 50% of chicken population in Nigeria and are capable of scavenging around the environment spreading the NDV and other avian viral diseases to vaccinated and unvaccinated healthy exotic birds [14,15,16]. This study recorded an overall seroprevalence of 93.3% in the study area. This prevalence rate is higher than the prevalence reported by [12] where they reported a seroprevalence of 17% in live bird markets in Abuja. However, this prevalence is also higher than the 32.5% prevalence rates reported by [17] in a study conducted on local chickens of live bird markets and households in Zamfara State, Nigeria [18] also reported a prevalence rate of 25.6% in live bird markets in Kogi State. This finding of NDV seropositivity in these apparently healthy birds suggests that the birds have either recovered from clinical ND or are having subclinical infections [19,20]. This study recorded variation in seroprevalence of NDV antibodies between the different avian species in live bird market in the study area with highest prevalence rates of 96 % in Broilers, followed by local chickens and guinea fowls with 92 % prevalence each. The difference in seroprevalence among the different Species was not statistically significant P>0.05. These findings agreed with studies conducted by [21,17] who reported a locational variation in NDV antibodies in the studies they carried out in local chickens from live bird markets and households in Zamfara State, Nigeria and rural household chickens in Plateau State, Nigeria respectively. Other studies, such as those by [18,22,17,23], revealed seroprevalence rates of 96%, 35.8%, 25.5%, and 65.1% respectively. The differences between the results of this study and those of [18,22,17,23], was that the latter collected samples from village chickens in live bird markets, where birds were known to be brought from various locations, including neighboring states, and congregate for sales.
From the table, the overall NDV seroprevalence of 96% obtained was higher than the result obtained by [24] in a retrospective study where they reported an NDV seroprevalence of 80.9% for chicken in Sokoto State. This may be due to the seasonality of NDV having high occurrence in the months of March and October which coincide with the onset of the rainy season and dry season, respectively. The high wind movement transfers infection from one poultry house or flock to another [21].
The ND antibodies detected in the local chickens and guinea fowls might have been as a result of natural exposure to the ND virus, since both had no history of previous vaccination against ND. But ND antibodies detected in the broilers might been as a result of vaccination against ND.
Manure from poultry litter is generally used as fertilizer and applied to fields for growing agricultural commodities [25]. Sokoto State, is known to have a large concentration of Onion and Garlic farmers. These agricultural products are grown using organic manure from poultry litters, which could be another reason for the significantly higher seroprevalence rate observed among the village chickens sampled in the Sokoto, where high concentration of commercial poultry farms is raising. This is because ND viruses are frequently discharged in high amounts in poultry faeces [26].
Village chickens are known to scavenge the environment for food, and ingestion of faeces during such activity could be a major route of transmission of ND virus among them [27].
Village chickens are naturally resistant and can withstand the infection without showing any clinical symptoms, thus acting as potential source of infection for commercial chicken. This means that village chickens act as host/ carrier of NDV; thus, chickens raised in the backyard of farm workers could increase the threat of ND outbreaks [28]. Management practices such as disease monitoring programme, appropriate prevention, and control measures should be put in place in order to prevent loss of poultry and income due to outbreaks of the disease. New birds should be quarantined, and local poultry farmers should ensure that they vaccinate their flocks [21]. Most importantly, an awareness programme among the farmers about the disease and routine survey to assess the degree of Newcastle disease distribution will help in planning an appropriate intervention strategy. The result of studies by [5] showed that the prevalence of NDV shedding was higher in village chickens than other breeds of commercial chickens.
Conclusion
Newcastle disease virus (NDV) antibodies were detected in broilers, local chickens and guinea fowls sold in live bird market in Sokoto metropolis the study area. They are likely to serve as host/carrier of NDV to commercial flocks as well as local poultry population. There is no statistically significant difference between antibodies of different types and breeds tested.
References
1. Alexander, D.J (1997). Newcastle disease and other avian Paramyxoviridae infections. In: Calnek BW, Barnes HJ, Beard CW, McDougald LR, Saif YM (ed), Disease of poultry, 10th edn. Iowa State University Press, Ames Iowa, pp 541–570.
2. Barbezange, C. and Jestin, V. (2005). Molecular study of the quasispecies evolution of a typical pigeon paramyxovirus type 1 after serial passages in pigeons by contact. Avian pathology 34: 111 – 122.
3. Dortmans, J. C., Koch, G., Rottier, P. J., and Peeters, B. P. (2011). Virulence of Newcastle disease virus: what is known so far?. Veterinary research, 42(1), 122. https://doi.org/10.1186/1297-9716-42-122
4. ClaudiaMarin, M., Villegas, P., Bennet, J.D. and Seal, B.S. (1996). Virus characterization and sequence of the fusion protein gene cleavage site of recent Newcastle disease virus field isolates from the Southeastern United States and Puerto Rico. Avian Disease 40: 382-390.
5. Sajo M.U., Hamisu, T.M., Saidu, A.S., Haruna, N.M., Shettima, Y.M., El-Yuguda, A.D., Abubakar, M.B., Madu, E.D., Waziri, M.M.(2023). Comparative Analysis of Newcastle Disease Virus Shedding from Naturally Infected Breeds of Poultry in Maiduguri, Nigeria. Sahel Journal of Veterinary Sciences Vol. 20, No. 3, Pp 33-37 http://doi.or10.54058/saheljvs.v20i3.380.
6. Pazhanivel, N., Balsubramaniam G.A, George, V.T. and Mohan, B. (2002). Study of natural outbreak of Newcastle disease in and around Namakkal. Indian Veterinary Journal 79(3): 293-294.
7. Solomon, P., Abolik, C., Joanis, T. and Bisschops, M. (2012). Virulent Newcastle disease virus in Nigeria: Identification of a new clade of sublineage 5F from live bird markets. Virus Genes, 44: 98-103
8. Unigwe, C.R., Shobowale, O.M., Enibe, F., Ajayi J.O., & Koleosho, S.A. (2020). Sero-prevalence of Newcastle disease in apparently healthy normal feathered local chickens in Ido and Atiba local government areas, Oyo State, Nigeria. AgroSciences Journal of Tropical Agriculture, Food, Environment and Extension, 19(4): 37 – 42.
9. Chang, A. and Dutch, R.E. (2012). Paramyxovirus fusion and entry: multiple paths to a common end. Viruses 4: 613-636.
10. Sokoto, B. A. (2001). The Study of Local Geography of Nigerian Secondary School, First edition, Ministry of Education, Sokoto.104-1.
11. Alkali, B. R., Shuaibu, A. B., Bello, I. and Usman, M. D., (2017). Seroprevalence of New Castle Disease Virus in Local Chickens from Sokoto, Nigeria Microbiology Research Journal International, 19(1): 1-5.
12. Abraham-Oyiguh, J., Sulaiman, L. K., Meseko, C. A., Ismail, S., Suleiman, I., Ahmed, S. J., and Onate, E. C. (2014). Prevalence of Newcastle Disease Antibodies in Local Chicken in Federal Capital Territory, Abuja, Nigeria. International Scholarly Research Notices, 2014: 796148.
13. Thrustfield VM. (2005)Veterinary epidemiology. 3rd Edition, Blackwell Science Ltd, Oxford, UK.p.183.
14. Abraham-Oyiguh, J., Sulaiman, L. K., Meseko, C. A., Ismail, S., Suleiman, I., Ahmed, S. J., & Onate, E. C. (2014). Prevalence of Newcastle Disease Antibodies in Local Chicken in Federal Capital Territory, Abuja, Nigeria. International scholarly research notices, 2014, 796148. https://doi.org/10.1155/2014/796148.
15. Food and Agricultural Organization (FAO) (2018). Livestock and livelihoods spotlight NIGERIA: cattle and poultry sectors. Food and Agriculture Organization of the United Nations, 1–12.
16. Bitrus, I., Shittu, I., Meseko, C. A. and Joannis, T. M. (2020). Occurrence and molecular detection of avian coronavirus in selected live bird markets, northwestern, Nigeria. Sokoto Journal of Veterinary Sciences, 18(4): 226-229.
17. Jibril, A.H., Umoh, J.U. and Kabir, J. et al. (2014). “Newcastle disease in local chickens of live bird markets and households in Zamfara State, Nigeria,” ISRN Epidemiology, vol. 2014, Article ID 51391, 4 pages .
18. Ameji, O. N., Abdu, P. A. and Saidu L. (2011). “Seroprevalence of Avian influenza, Newcastle disease and Gumboro disease in chicken in Kogi State, Nigeria,” Bulletin of Animal Health and Production African Journal, 59:4.
19. Adu, F. D., Edo, U. and Sokale, B. (1986). Newcastle Disease: the immunological status of Nigeria Local Chickens.Tropical Veterinary, 4:149-152.
20. Ameh, J. A., Mailafia, S., Olabode, O. H., Adah, B. J., Okoh, G. R., Ogbole, M. E. and Alalade, D. I. (2016). Sero-prevalence of Newcastle disease virus antibodies in local and exotic chickens in Gwagwalada, Nigeria. Journal of Veterinary Medicine and Animal Health, 8:193–198.
21. Musa, U., Abdu, P. A., Dafwang I.I. et al., (2009) “Seroprevalence, seasonal occurrence and clinical manifestation of Newcastle disease in rural household chickens in plateau state, Nigeria,” International Journal of Poultry Science, vol. , no. 2, pp. 200–204.
22. Chollom, S.C, Emerhirhi, F.T, Akwaowo, E.E, Ogbaji, J.U and Fyaktu, E.J (2013). Implication of Newcastle disease virus in local chickens at live bird markets in Jos. Nigeria. International Journal of Current Research and Review, 5(14): 28722874.
23. Eze, I.A & Ike, A.C (2015). The serological status for Newcastle disease in local chickens of live bird markets and households in Nsukka, Enugu State, Nigeria. Nigeria Journal of Microbiology, 29(2): 3096-3104
24. Ibitoye, E. B. Jimoh, A. A. and Mungadi, H. U.(2013) “A retrospective (2007–2011) analysis of Newcastle disease diagnosed at Avian clinic of veterinary teaching hospital, Usmanu Danfodiyo University Sokoto, Nigeria,” Current Research in Poultry Science, vol. 3, no. 1, pp. 12– 17, 2013.
25. USPEA (1998). Environmental framework and implementation strategy for poultry operations.United State Poultry and Egg Association.
26. Alexander, D.J, Wilson, G.W.C, Russell P.H, Lister, S.A. and Parsons, G. (1985). Newcastle disease outbreaks in fowl in Great Britain during 1984, Veterinary Records, 117(17): 429-434.
27. Sa’idu, L., Abdu, P.A., Tekdek, L.B., Umoh, J.U., Usman, M. and Oladele, S.B. (2006). Newcastle disease in Nigeria. Nigerian Veterinary Journal, 27(2): 23-32.
28. Ananth, R., Kirubaharan, J. J., Priyadarshini, M. L. M. and Albert, A. (2008) “Isolation of Newcastle disease viruses of high virulence in unvaccinated healthy village chickens in South India,”International Journal of Poultry Science, vol. 7, no. 4, pp. 3–373, 200.
This article licensed under the Creative Commons Attribution 4.0 International License CC-BY 4.0., which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are properly credited.
