NL Journal of Agriculture and Biotechnology
(ISSN: 3048-9679)
Chlorophyll Content of Groundnuts [Arachis hypogaea (L.)] Grown on a P– deficient Samaru Alfisols as Affected by Mycorrhizal Inoculation and Phosphorus Application
Author(s) : A. I. Gabasawa, A. S. Kona. DOI : 10.71168/NAB.01.01.108
Abstract
A pot experiment was conducted in the Department of Soil Science screen house, Ahmadu Bello University, Zaria. It was to determine the effects of mycorrhizal inoculation and phosphorus (P) application on chlorophyll contents of some groundnut genotypes. The treatment consisted of five groundnut genotypes (ARRORS-ICGX 000201/5/P4/P10, ICGX-SM 00010/5/P15/P1, ICGV-IS 07083, Kwankwaso and SAMNUT 24), four levels of P (0, 20, 40 and 60 kg P2O5 ha-1) and two levels of (with and without) mycorrhizal inoculation. These combinations were laid out in a randomized complete block design and replicated thrice. Data observed included: chlorophyll content, plant height and numbers of branches, leaves and flowers. The soil was initially analysed for some physical and chemical properties. The genotype ICGV-IS 07083 significantly (p<0.01) produced tallest plants. It also emerged second and third in terms of number of branches and numbers of leaves and flowers respectively. However, SAMNUT 24 and ICGX-SM 00010/5/P15/P1 had the highest number of flowers. Similarly, the inoculated (WiM) genotypes significantly (p<0.01) produced the tallest plants than the non-inoculated (WoM) but had the highest leaf number. There was no significant difference between the WiM and WoM in terms of numbers of branches and flowers. Application of 0 and 60 kg P2O5 ha-1 significantly (p<0.01) produced the highest number of flowers. Notwithstanding, P levels were at par in terms of plant height. SAMNUT 24 had the highest value of chlorophyll content (45.5 SCMR). The high yield observed in the inoculated SAMNUT 24 and without P application, is attributable to the chlorophyll content and is therefore, recommended for a profitable resource-poor farmer production programme under limited soil P conditions. Keywords: Alfisols, Chlorophyll content, Mycorrhizal inoculation, Phosphorus, SAMNUT
Introduction
Groundnut (Arachis hypogaea (L.)) is an important oil seed crop in Nigeria and widely grown in the tropics and sub – tropics [1]. It is one of the most important crops that have the ability to thrive on newly reclaimed sandy soil as a legume of high nutritive value [2]. It plays a vital role in the economy by reducing the need for mineral nitrogen (N) fertilizer [3] as It was estimated to fixed 72 to 297 kg N ha-1 [4]. Arbuscular mycorrhizal fungi is considered to be widely symbiotic association between specific soil fungi and a plant root [5] as the fungus banks on the plant host for carbon in and in return the fungus improves nutrition especially phosphate nutrition [6].
Chlorophylls are important pigments responsible for converting solar energy into chemical energy that is used to build essential carbohydrate molecules (glucose) which are used as food source for the whole plant [7]. Phosphorus is an essential plant macronutrient which is required to build important molecules such as nucleic acids and phospholipids and plays a central role during energy transfer in processes like nicotinamide adenine dinucleotide phosphate (NADPH), Adenosine Triphosphate (ATP) and regulation of enzymatic and metabolic reactions [8,9]. The free phosphate levels available to plant around the soil are used to be very low and may range from less than 1 to 10 µM [10]. Phosphorous typically constitutes around 30 %, 65 % or 80 % of total P in the soil as organic P [11] but still the availability of 80–99 % phosphorous for uptake in plant is scarce due to different factors like adsorption, precipitation or conversion into organic forms [6]. Hence, P-deficiency is a factor limiting groundnut production on tropical and subtropical soils [12]. It has been shown that low level of soil phosphorus reduces nodulation [13]. Mycorrhizal association is of significant importance for the P supply since the extra-radical hyphae of AMF act as root extensions and draw P from soil to supply it to plants [14]. Many researchers have reported an increase in P concentration in mycorrhizal plants [15]. The transfer of P to plants by AMF is influenced by P addition to soils, as it decreases the mycorrhizal association [16]. Moreover, plants take up inorganic phosphorus (Pi) from the soil as orthophosphate ions [17] due to which there may be a decreased P in soil, especially around the roots but extra radical hyphae of AMF grow beyond this depletion zone and provide positive effects on plants [6]. The aim of this research was to determine the chlorophyll content of groundnuts [Arachis hypogaea (L.)] grown on a P–deficient Samaru Alfisols vis-à-vis the influence of mycorrhizal inoculation and P application.
Materials and Methodology
Site and Description
The study was carried out on an Alfisols sampled from the experimental research farm of the Institute for Agricultural Research (IAR) Samaru-Zaria (lat. 110 10’ N, long. 7036’ E) with an altitude of 704 m above sea level in the Northern Guinea Savannah of Nigeria. The soil of the area has been described as leached tropical ferruginous, classified as Typic Haplustalf in soil taxonomy, Acrisol in the FAO system, or Alfisols in the USDA system [18].
Collection and Preparation of Treatments
Soil Sampling and Preparation
A composite surface soil (0-15 cm) and sub-surface samples (15–30 cm) were taken from the experimental site. It was thoroughly mixed, air-dried, crushed and passed through 2 mm stainless sieve. The less than 2 mm fraction was used for some physicochemical laboratory analyses. Another soil was sieved through a 6 mm mesh, which was used for the pot experiment. The pot cover was placed underneath to collect the possibly drained water and dissolved nutrients through the holes made at the bottom of the pot which were appropriately returned into the pots.
Treatments and Experimental Design
The treatments were five (5) different genotypes (ARRORS ICGX 000201/5/P4/P10, ICGX-SM 00010/5/P15/P1, ICGV-IS 070830, Kwankwaso and SAMNUT24 and four (4) levels of phosphorous (at 0, 20, 40 and 60 kg P2O5 ha-1) with mycorrhiza (Mycodrip). It was prepared at a 1: 9 inoculums: sterilized sand ratio; the inoculants mixed with sterilised sand were applied at the centre of the holes where seeds were sown and without mycorrhizal (WoM) inoculants. The pots were arranged in randomized complete block design (RCBD) with three replications.
The soils were watered and allowed to attain field capacity before sowing. After sowing the pots were watered based on plant need until harvest. There were, therefore, 40 treatment combinations per replication which together gave 120 pots, as indicated in Table 1. All treatments were randomly allocated.
Table 1. showing the treatment combinations used per replication
| S/No. | Treatment Combinations | S/No. | Treatment Combinations |
| 1. | G1P0WiM | 21. | G3P40WiM |
| 2. | G1P0WoM | 22. | G3P40WoM |
| 3. | G1P20WiM | 23. | G3P60WiM |
| 4. | G1P20WoM | 24. | G3P60WoM |
| 5. | G1P40WiM | 25. | G4P0WiM |
| 6. | G1P40WoM | 26. | G4P0WoM |
| 7. | G1P60WiM | 27. | G4P20WiM |
| 8. | G1P60WoM | 28. | G4P20WoM |
| 9. | G2P0WiM | 29. | G4P40WiM |
| 10. | G2P0WoM | 30. | G4P40WoM |
| 11. | G2P20WiM | 31. | G4P60WiM |
| 12. | G2P20WoM | 32. | G4P60WoM |
| 13. | G2P40WiM | 33. | G5P0WiM |
| 14. | G2P40WoM | 34. | G5P0WoM |
| 15. | G2P60WiM | 35. | G5P20WiM |
| 16. | G2P60WoM | 36. | G5P20WoM |
| 17. | G3P0WiM | 37. | G5P40WiM |
| 18. | G3P0WoM | 38. | G5P40WoM |
| 19. | G3P20WiM | 39. | G5P60WiM |
| 20. | G3P20WoM | 40. | G5P60WoM |
G1-5 = Genotypes 1 - 5, P0, 20, 40 and 60 = P levels at 0, 20, 40 and 60 kg P2O5 ha-1, WiM = with mycorrhizal and WoM = without mycorrhizal inoculant.
Preparation of Micronutrients
Micronutrients were prepared according to Vincent (19 1970) and applied at the rate of 1 ml kg-1 of the soil at three weeks after sowing. The composition of the micronutrients was as stated in Table 2.
| S/No. | Salt | Quantity (g) |
| 1. | H3BO3 | 2.78 |
| 2. | MnSO4.7H2O | 1.54 |
| 3. | ZnSO4.7H2O | 0.21 |
| 4. | Na2MnO4 | 4.36 |
| 5. | FeCl2.6H2O | 5.00 |
| 6. | CaSO4.6H2O | 0.004 |
| 7. | Lactic Acid (88 %) | 580 ml |
| 8. | Distilled Water | 420 ml |
Addition of 1.0 ml L-1 of medium gives: B, 0.5µg; Mn, 0.5 µg; Zn, 0.05 µg; Mo, 0.1 µg; Fe, 100 µg; and Co, 0.0005 µg per litre (or ppm) [19].
Laboratory Analyses
The soil sample collected from the experimental site was analysed for the following physical and chemical parameters: particle size distribution using hydrometer method; [20] soil reaction (pH) in 1:2.5 soil to water and 0.01 M CaCl2 ratio suspension with the glass electrode pH meter as described by Agbenin [21]. The organic carbon was determined using the modified Walkley-Black methods described by Anderson and Ingram [22]. Total Nitrogen was determined by Micro-Kjehldahl technique [23] and Available P by Bray 1 method according to Bray and Kurtz [24]. The Exchangeable bases (Na, K, Ca and Mg) were, however, determined by the ammonium acetate (NH4OAc) method based on procedure by Anderson and Ingram [22]. Sodium (Na) and K were determined using Flame Photometer and Ca and Mg were both determined using Atomic Absorption Spectrophotometer (AAS). Soil samples were, also on the other hand, analysed. They were analysed for Total N; by micro - Kjehdahl method [23] . Available P was determined using Bray 1 method [24], and K; following the NH4OAc procedure [22].
Determination of Root Colonisation
Freshly harvested groundnut roots from the screen house were taken to laboratory and stored in a 50% ethanol before the mycorrhizal study. Methods described by Dexheimer et al. [25] and McGonigle et al.[26] were employed for this study. The roots, earlier stored, were washed with distilled water and then heated in a prepared 10% KCl for 20 min at 900C after which they were rinsed with tap water. They were then placed in a 10 ml trypan blue solution over night to absorb the colour. The roots were then put, overnight, in a destaining solution (50% glycerol) on a microscope slide beneath a 50 x 22 mm cover slip. The roots were mounted as parallel lengths so as to avoid tangles, which will otherwise make quantification very difficult [27]. The roots finally washed with water and examined under the microscope at 10 x magnifications. Twenty-five root bits, earlier sampled, were arranged on each slide for each treatment. Each root bit was observed under the microscope for the presence of any arbuscular mycorrhizal fungal structure, and if present, was marked as colonised. Then the percentage root colonisation was therefrom calculated as follows:
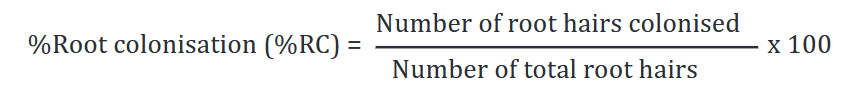 Statistical Analysis
Statistical Analysis
All data collected were subjected to analysis of variance (ANOVA) using the statistical analysis system (SAS) statistical computer package [28]. Means with significant effect at 5% level of probability were separated using the Duncan’s multiple range test (DMRT). Parameters with coefficient of variability (CV) above the acceptable limit of 40% were transformed following the log transformation procedure [29,30].
Results and Discussion
Soil Analyses
The physical and chemical properties of the soil at 2 depths (0-15 cm and 15-30 cm) were determined (Table 1). The result on particle size analysis showed a proportion of 54, 25 and 21 % for sand, silt and clay respectively at 0-15 cm and 62, 17 and 21 % at 15-30 cm giving the textural class of sandy clay loam). The organic matter (6.8 and 7.2 g kg-1) and organic carbon (0.35 and 0.42 g kg-1) contents were low at 0-15 cm and 15-30 cm depths respectively as the organic matter values fall within the low (> 20 g kg-1) category, according to Federal Ministry of Agriculture and Natural Resources (FMANR) [31]. This could be as a result of the sandy nature of the soil and low rate of organic residues. This encourages rapid leaching of cations and consequent low cation exchange capacity (CEC) values [32,33]. The soil reaction was slightly acidic both in water (5.9 and 6.1) and in CaCl2 (4.8 and 4.5) at 0-15 cm and 15-30 cm respectively. The pH values in water were higher than those in salt indicating the possession of a net negative charge in the soil colloidal complex [34]. The value of available P was quite low (i.e., within the low range value of 0 – 10 mg kg-1) according to Esu [35]. Total N content and exchangeable K were also low, indicating poor nutrient reserve of the soil when compared with the ranges of Esu [35]. The value of Na obtained from the result was 0.53 cmol kg-1, that of Mg was 0.77 cmol kg-1 and Ca was 2.6 cmol kg-1 all of which fall under low level range according to FMANR [31], which is certain to lead to low yields.
Table 3. Showing result of soil characterization before sowing
| Parameter | Unit | Depth (0 – 15 cm) | Depth (15 - 30 cm) |
| Particle size distribution | (g kg-1) | ||
| Sand | 540 | 620 | |
| Silt | 250 | 170 | |
| Clay | 210 | 210 | |
| Textural class | Sandy clay loam | Sandy clay loam | |
| Soil reaction (pH) | |||
| water (1:2.5) | 5.90 | 6.10 | |
| CaCl2 (1:5) | 4.80 | 4.50 | |
| Organic C | g kg-1 | 0.30 | 0.40 |
| Organic matter* (g kg-1) | g kg-1 | 6.80 | 7.20 |
| Total N (g kg-1) | g kg-1 | 0.65 | 0.45 |
| Exchangeable Bases | cmol(+) kg-1 | ||
| K | 0.50 | 0.19 | |
| Na | 0.53 | 0.15 | |
| Ca | 2.60 | 2.40 | |
| Mg | 0.77 | 0.72 | |
| CEC | 7.50 | 10.20 |
*OC (g kg-1) x 2 [36]
Effects of genotype, P and mycorrhizal inoculation on plant height and numbers of branches, leaves and flowers of the genotypes
Table 4 shows the results obtained from the statistical analysis of the genotype, P levels and mycorrhiza on chlorophyll contents, plant height and numbers of branches, leaves and flowers of the genotypes. There was a significant difference among the genotypes on chlorophyll contents (p<0.01), plant height (p<0.01) and numbers of branches (p<0.01), leaves (p<0.01) and flowers (p<0.01). The genotypes ARRORS ICGX 000201/5/P4/P10, ICGV-IS 07083 and SAMNUT 24 were statistically similar and had significantly highest chlorophyll content. These were immediately followed by ICGX-SM 00010/5/P15/P1 and Kwankwaso, which were statistically similar and recorded the least chlorophyll content. This indicated that the genotypes ARRORS ICGX 000201/5/P4/P10 and ICGV-IS 07083 had greater potentials for yield as more N2-fixation will be expected from them due to their response to mycorrhizal inoculation and P application. The genotype ICGV-IS 07083 had the tallest plants and was followed by Kwankwaso and SAMNUT 24 both of which were also statistically at par with ICGX-SM 00010/5/ P15/P1 and ARRORS ICGX 000201/5/P4/P10. These two also had the numerically least height. This, respectively, made them best and better responders to the inoculation and P application of the other genotypes. The genotype Kwankwaso recorded the highest number of branches followed by genotype ICGV-IS 07083 which was statistically at par with SAMNUT 24. The genotype ICGX-SM 00010/5/P15/P1 was also at par with ARRORS ICGX 000201/5/ P4/P10 and both recorded the least branch number. Nomadic and other livestock keepers can, therefore, reap the advantage of growing these genotypes provided they will inoculate. Also, Kwankwaso recorded the highest number of leaves, and it was followed by SAMNUT 24, ICGV-IS 07083 and ICGX-SM 00010/5/P15/P1 which were statistically similar. ARRORS ICGX 000201/5/P4/P10, however, recorded the least leaves numbers. The genotypes ICGX-SM 00010/5/P15/P1 and SAMNUT 24 were statistically similar, and each recorded the highest number of flowers. ARRORS ICGX 000201/5/P4/P10 followed those while ICGV-IS 07083 and Kwankwaso were at par and each recorded the least number of flowers.
The effect of P on chlorophyll contents, plant height and numbers of branches and leaves was not significant (p>0.05). However, it was significant in number of flowers (p<0.01) in which the P levels of 0 and 60 kg P2O5 ha-1 were statistically at par gave significantly highest number of flowers. These were immediately followed by the P levels of 20 and 40 kg P2O5 ha-1 which were also statistically similar. The treatments with mycorrhizal inoculation (WiM) produced significantly (p<0.01) tallest plants. This could be due to effect of mycorrhizal colonisation which is of an essential importance to leguminous growth and development. Mycorrhizal colonisation is known to increase P acquisition of an otherwise restricted root system which usually leads to poor soil P acquisition by crop plants [37]. The treatment without mycorrhizal inoculation (WoM), however, produced significantly (p<0.01) highest number of leaves. The P applied may possibly be at play here. There was, however, no significant (p>0.05) difference, in terms of the chlorophyll contents; and numbers of branches and flowers produced by the genotypes.
The results of the interactions of genotype, P and mycorrhiza on plant height; and numbers of branches, leaves and flowers were as shown on Table 2. The genotype by mycorrhiza interaction significantly (p<0.01) influenced only the number of branches not (p>0.05) the plant height, number of leaves or flowers. The genotype by P interaction significantly influenced the numbers of branches, leaves and flowers (p<0.01) but not the plant height (p>0.05). This is in corroboration with the findings of many authors, including: Kogbe et al [38]. Lekberg and Koide [39], Ibrahim and Eleiwa [40] and Gabasawa [3]. The mycorrhiza by P interaction also significantly (p<0.01) influenced the number leaves recorded for the genotypes. However, there was no significant (p>0.05) difference in terms of interaction on plant height, number of branches or number of flowers recorded. There was also no significant (p>0.05) difference in genotype by mycorrhiza by P interaction on any of plant height or numbers of branches, flowers and leaves.
Interaction between genotype and mycorrhiza on number of branches of the genotypes
The result on the interaction of genotype and mycorrhiza on number of branches as observed in (Figure 1) shows that the interaction was significant (p<0.01) and further revealed that the genotype Kwankwaso WiM recorded highest number of branches than any other genotype, both WiM and WoM, followed by genotype ICGV-IS 07083 WoM genotypes ICGX-SM 00010/5/P15/P1 and SAMNUT 24 WiM recorded highest number of branches.
Table 4: Effects of Genotype, P and Mycorrhiza on Chlorophyll Content, plant height, number of branches, leaves and flowers of the genotypes
| Treatments | Chlorophyll
Content (SCMR) |
Plant Height | No. of
Branches (plant-1) |
No. of leaves (plant-1) | No. of flowers (plant-1) |
| Genotype (G) | |||||
| ARRORS ICGX 000201/5/
P4/P10 |
42.51a | 26.43c | 31.08d | 129.67c | 21.08b |
| ICGX-SM 00010/5/P15/P1 | 41.30bc | 27.81c | 34.88cd | 140.71bc | 30.17a |
| ICGV-IS 07083 | 43.01a | 31.92a | 41.21b | 152.08b | 17.46c |
| Kwankwaso | 39.95c | 30.92bc | 46.91a | 179.75a | 13.96c |
| SAMNUT 24 | 43.55a | 28.75bc | 37.41bc | 152.92b | 28.91a |
| SE+ | 0.521 | 0.641 | 1.540 | 5.132 | 1.261 |
| Phosphorus Level (P) | |||||
| 0 | 42.05 | 28.36 | 37.63b | 142.43b | 23.60a |
| 20 | 41.64 | 28.82 | 35.80b | 156.13a | 20.10b |
| 40 | 42.04 | 29.94 | 38.20ab | 158.10a | 20.20b |
| 60 | 42.52 | 29.55 | 41.59a | 147.43ab | 25.37a |
| SE+ | 0.471 | 0.750 | 1.371 | 4.590 | 1.131 |
| Mycorrhiza (M) | |||||
| WiM | 42.02 | 29.59a | 37.78 | 48.52b | 21.82 |
| WoM | 42.10 | 28.74b | 38.82 | 158.63a | 22.82 |
| SE+ | 0.333 | 0.531 | 1.123 | 3.240 | 0.832 |
| Interactions | |||||
| G x P | NS | NS | * | NS | NS |
| G x M | NS | NS | * * | ** | * |
| P x M | NS | NS | NS | * | NS |
| G x P x M | NS | NS | NS | NS | NS |
SCMR = Spad-Chlorophyll Meter Reading, WoM = Without, WiM = with mycorrhiza, NS = Not significant at 5% level of probability, ** = significant at 1 % level of probability, means with the same letter(s) within a column are not significantly different by DMRT.
There was no significant difference in both WiM and WoM in genotype ARRORS ICGX 000201/5/P4/P10.
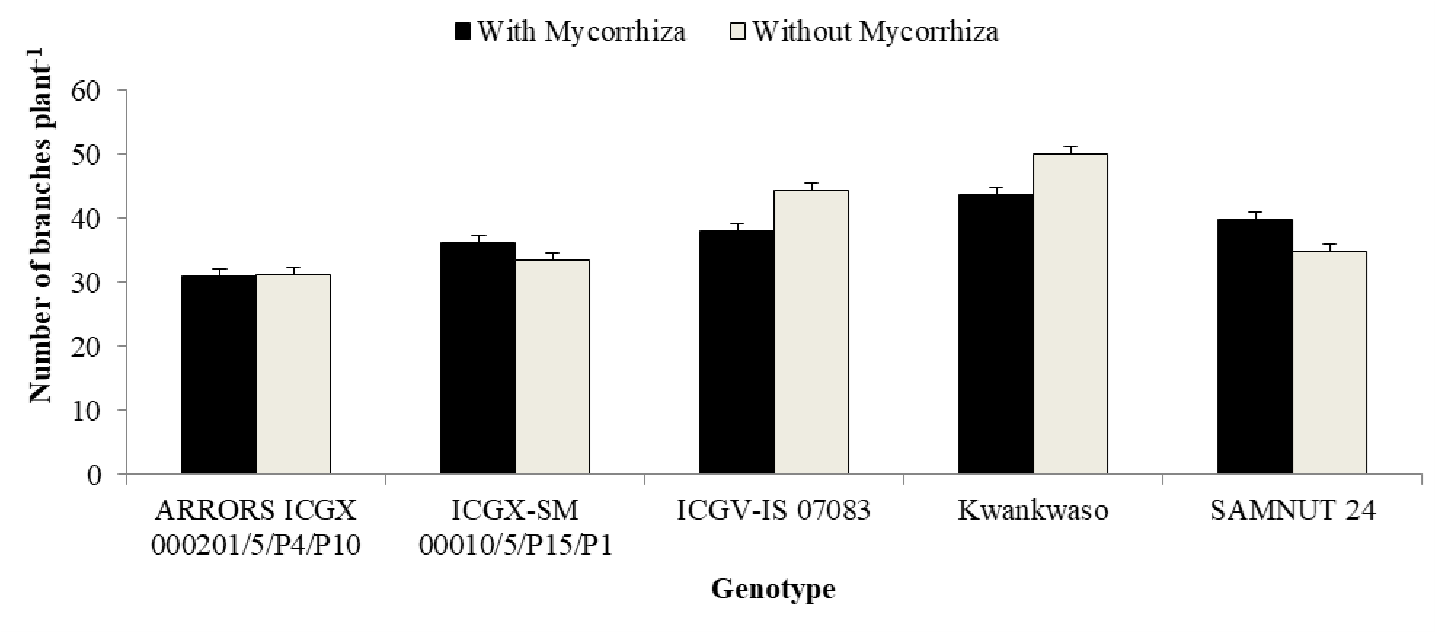
Figure 1. Interaction between genotype and mycorrhiza on number of branches of the genotypes
Interaction between genotype and P on number of branches of the genotypes
There was a significant (p<0.01) interaction between the genotype and P in terms of number of branches (Figure 2). The highest number of branches was recorded at the highest P level (60 kg P2O5 ha-1) by Kwankwaso followed by genotype Kwankwaso at P level of (0 kg P2O5 ha-1). All other genotypes were statistically similar in terms of number of branches.
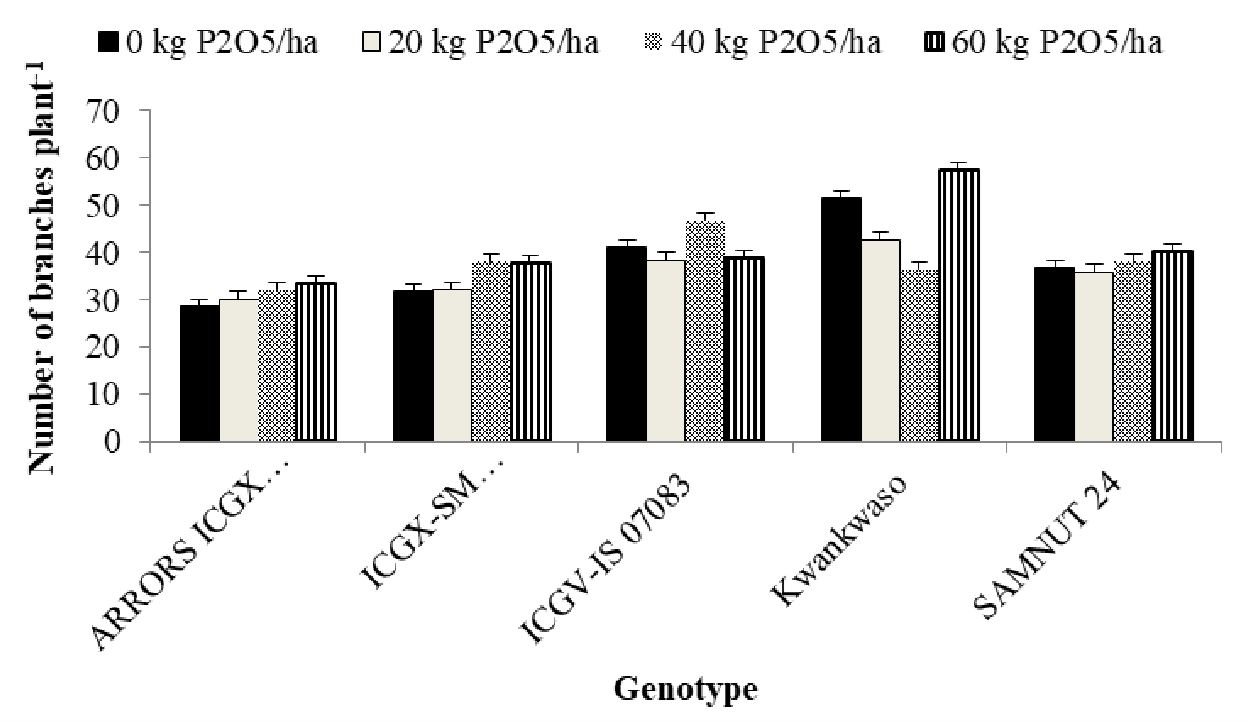
Figure 2. Interaction between genotype and P on number of branches of the genotypes
Interaction between genotype and P on number of leaves of the genotypes
The interaction between genotype by P in terms of leaves number showed significant (p<0.01) difference as shown in (Figure 3). The highest number of leaves, than other P levels, was recorded at 20 kg P2O5 ha-1 P level) by genotype Kwankwaso followed by the 0 kg P2O5 ha-1 level of P with the same Kwankwaso genotype. All the rest were statistically at par in number of leaves.
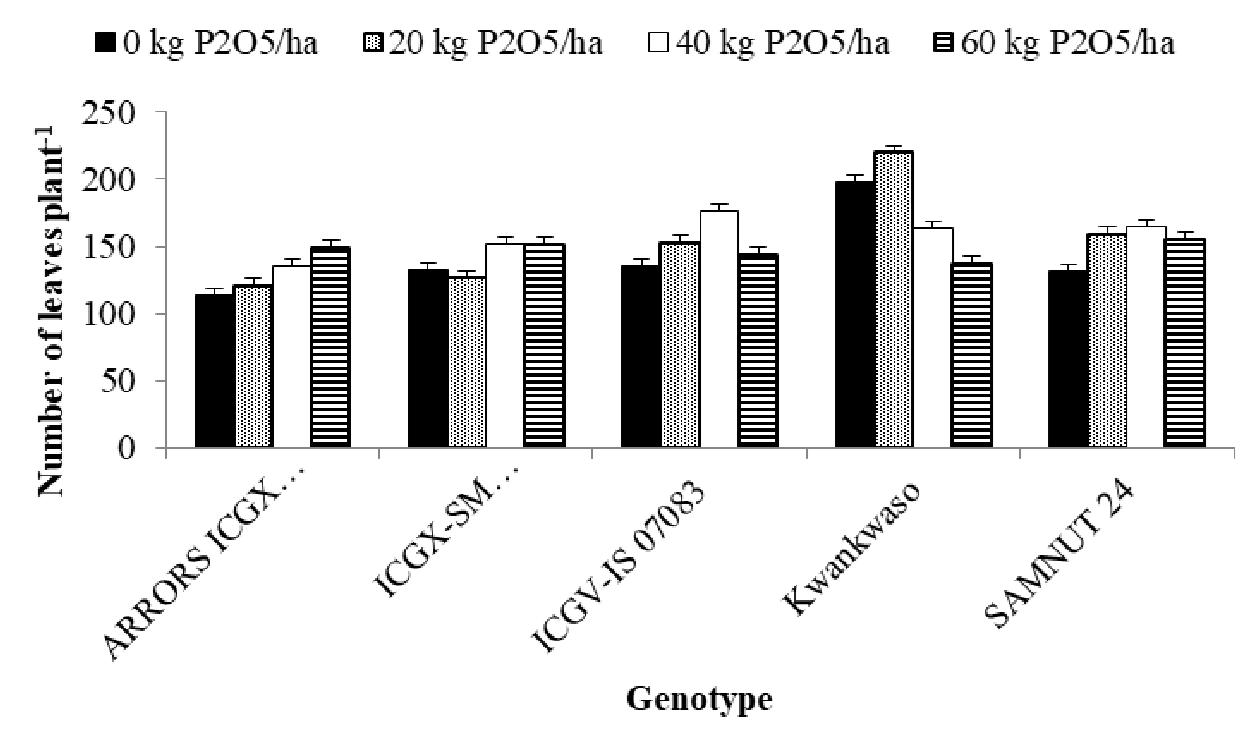
Figure 3. Interaction between genotype and P on number of leaves of the genotypes
Interaction between genotype and P on number of flowers of the genotypes
The interaction between genotype and P, in terms of number flowers, showed significant (p<0.01) difference (Table 5). The highest number of flowers was recorded at the least P level (0 kg P2O5 ha-1) by ICGX-SM 00010/5/ P15/P1 and highest P level (60 kg P2O5 ha-1) by SAMNUT 24, both of which were statistically at par and significantly higher than all other genotypes, in terms of number of flowers. These were immediately followed by same ICGX-SM 00010/5/P15/P1 at 60 kg P2O5 ha-1 level and SAMNUT 24 at 0 and 40 kg P2O5 ha-1 P levels, which were statistically similar. These were followed by the statistically at par rest of genotypes. This indicated that the two genotypes (ICGX-SM 00010/5/P15/P1 and SAMNUT 24) can be relied - upon, for a high yielding production programme, under limited soil P conditions, as good number of flowers can be used in predicting potential yield of groundnuts.
Table 5. Interaction between genotype and P on number of flowers of the genotypes
| Genotype | P level (kg P2O5 ha-1) | |||
| 0 | 20 | 40 | 60 | |
| ARRORS ICGX 000201/5/P4/P10 | 19.83e-h | 20.17e-h | 21.17d-g | 23.17c-e |
| ICGX-SM 00010/5/P15/P1 | 39.50a | 27.17c | 25.17cd | 28.83b |
| ICGV-IS 07083 | 16.00hi | 16.5g-i | 15.83hi | 21.50d-f |
| Kwankwaso | 14.00ij | 13.50ij | 10.50j | 17.83f-i |
| SAMNUT 24 | 28.67b | 23.17c-e | 28.33b | 35.50a |
| SE± | 2.530 | |||
Means with the same letter(s) within a column are not significantly different by DMRT.
Interaction between mycorrhiza and P on number of leaves of the genotypes
The result on the interaction between mycorrhiza and P on number of leaves as observed in (Table 6) showed that the P level at 20, kg P2O5 ha-1, and without mycorrhiza, recorded the highest number of leaves, even higher than 20, 40 and 60 kg P2O5 ha-1 P levels with mycorrhizal inoculation. Phosphorus applied at 0 and 40 kg P2O5 ha-1 levels without mycorrhiza were also statistically similar. Application of 60 kg P2O5 ha-1 without mycorrhiza recorded least number of leaves. The genotypes that received 20 kg P2O5 ha-1, and without mycorrhiza, would, therefore, be more economical for the resource-poor farmers for obvious reason.
Table 6. Interaction between mycorrhiza and P on number of leaves of the genotypes
| P level (kg P2O5 ha-1) | Mycorrhiza | ||
| WiM | WoM | ||
| 0 | 132.07b | 152.8bc | |
| 20 | 148.00cd | 164.27a | |
| 40 | 161.53ab | 154.67b-c | |
| 60 | 152.07b-d | 142.80d | |
| SE± | 6.488 | ||
WoM = without, WiM = with mycorrhiza, means with the same letter(s) within a column are not significantly different by DMRT.
Conclusion
It was observed d that the genotype ARRORS ICGX 000201/5/P4/P4/P10, ICGV-IS 07083 and SAMNUT 24 recorded highest chlorophyll content. Also, the WiM treatments recorded the highest chlorophyll content There was, however, no evidence of any significant role of P level of applications on the chlorophyll content of the genotypes. The genotype SAMNUT 24 is recommended for a resource-limited farmer among all other genotypes. This is due to its high yield potentials under limited soil P conditions, although requiring a a level of mycorrhizal inoculation ICGV-IS 07083 produced significantly tallest plant as well as the second number of branches while third on leaves and flowers. However, genotype ICGX-SM 00010/5/P15/P1 and SAMNUT 24 had the highest number of flowers. Similarly, the crop with Mycorrhiza inoculation significantly gave the highest plant height, while the crop without Mycorrhiza inoculation had the highest number of leaves. However, there was not significant different between WiM and WoM in terms of number of branches and flowers. The application of 60 kg P2O5 ha-1 significantly produced the tallest plant height, as well as highest number of flowers.
References
1. Nigam, S.N., S.L. Dwived and R.W. Gibbson, 1991. Groundnut breeding, constraints, achievements and future possibilities. Plant Breeding Abst. 61: 1127-1136.
2. Desire, T.V., M.T. Liliane, N.M. Le prince, P.I Jonas and A. Akoa, 2010. Mineral nutrient status, some quality and morphological characteristic changes in peanut (Arachis hypogaea L.) cultivars under salt stress. Afri. J. Environ. Sci. Technol., 4: 471-479.
3. Gabasawa, A.I. (2011). Varietal differences in phosphorus use efficiency and nitrogen fixation in groundnuts at Samaru, Nigeria. Unpublished MSc Thesis submitted to The School of Postgraduate Studies, Ahmadu Bello University, Zaria, Nigeria. https//:doi.org/10.13140/RG.2.2.32223.18087
4. Anuar, A.R., Shamsuddin, Z.H. and Yaacob, O. (1995). Contribution of legume-N by nodulated groundnut for growth of maize on an acidic soil. Soil Biol. Biochem.27: 595-601.
5. Schüßler, A., Schwarzott, D. and Walker, C. (2001). A new fungal phylum, the Glomeromycota, phylogeny and evolution. Mycol Res., 105: 1413-1421.
6. Smith, S.E. and Read, D.J. (1997). Mycorrhizal symbiosis. Second Edition. London: Academic Press, 1-605 p.
7. Hynninen P H, Leppakases T S. 2002. The functions of chlorophyll sinphotosynthesis: physiology and maintenance. In: Encyclopaedia of Life Support System, vol.5, pp.1–9. EOLSS: Oxford, UK.
8. Bieleski, R.L. and Ferguson, I.B. (1975). Physiology and metabolism of phosphate and its compounds. In: Encyclopaedia of Plant Physiology, Volume 5a, NY, Springer Verlag, 422-449 p.
9. Theodorou, M.E. and Plaxton, W.C. (1993). Metabolic adaptations of plant respiration to nutritional phosphate deprivation. Plant Physiol., 101: 339-344.
10. Marschner, H. (1995). Mineral nutrition of higher plants, 2nd Edition. London: Academic Press, 889 p.
11. Fageria, N.K. (2009). The use of nutrients in crop plants, 91-123 p. In: Taylor and Francis Gsroup, CRC press, Boca Raton, FL. Phosphorus.
12. Fairhurst, T.R., Lefroy, R., Mutert, E. and Batijes. N. (1999). The importance, distribution and causes of phosphorus deficiency as a constraint to crop production in the tropics. Agroforestry Forum 9(4): 2-8.
13. Caron, D.A, Goldman, J.C. and Dennett, M.R. (1986). Effect of temperature on growth, respiration by an omnivorous microflagellate 52: 1340 – 1347.
14. Ramos-Zapata, J., Orellana, R., Guadarrama, P. and Medina-Peralta, S. (2009). Contribution of mycorrhizae to early growth and phosphorus uptake by Neotropical palm. J Plant Nutr., 32: 855-866.
15. Yao, Q., Zhu, H.H., Hu, Y.L. and, Li, L.Q. (2008). Differential influence of native and introduced arbuscular mycorrhizal fungi on growth of dominant and subordinate plants. Plant Ecology. 196: 261-268
16. Vierheiling, H. (2004). Regulatory mechanisms during plant arbuscular mycorrhizal fungus interaction. Canad J Bot., 82(8): 1166-1176.
17. Holford, I.C.R. (1997). Soil phosphorus: Its measurement, and its uptake by plants. Aust J Soil Res., 35: 227 - 239.
18. Uyovbisere, E.O., Chude, V.O and Bationo A. (2000). Promising nutrient ratios in fertilizer formulations for optimal performance of maize in the Nigerian savannah. The need for a review of current recommendation. Nigerian Journal of Soil Research, 1; 29-34
19. Vincent, JM (1970). A manual for practical study of root nodule bacteria. IBP Handbook No. 15, Blackwell Scientific Publishers, Oxford, 164p.
20. Gee, G.W. and J.W. Bauder. (1986). Particle size analysis. In: A. Bluter (ed.) Methods of soil analysis part 2 (2nd edition) No. 9 ASA Inc. Madison, Washington, D.C.
21. Agbenin, J.O. (1995). Laboratotory manual for soil and plant analtsis. Selected methods and data analysis. Pp. 13-15.
22. Anderson, J.M. and Ingram, J.S.I. (1993). Tropical soil biology and fertility: A hand book of methods. London, CAB International.
23. Bremner, J.M. (1965). Total nitrogen. In: C.A. Black (Eds) Method of Soils Analysis. Agronomy No. 9 Part 2, Amer. Soc. Agronomy, Madison, Wisconsin.
24. Bray R.H. and Kurtz, L.T. (1945). Determination of total, organic and available forms of phosphorus in soil. Soil Science, 39-45.
25. Dexheimer, J. (1979). Enzymatic studies on the metabolism of vesicular-arbuscular mycorrhiza. 3. Ultrastructural location of acid and alkaline phosphatase activity in onion roots infected by Glomus mosseae (Nicol. & Gerd.). New Phytology 82,127-132.
26. McGonigle T.P., Miller M.H., Evans D.G., Fairchild D.G. and Swann J.A. (1990). A new method which gives an objectives measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. The new Phytologist. 155: 495 – 501.
27. Brundrett, M., Melville, L. and Peterson, L. (1994). Practical methods in mycorrhiza research. Mycologue publications. Guelph, Ontario, Canada.
28. Statistical Analysis System Institute. (2014). SAS 9.4. SAS Institute, Inc., Cary, NC.
29. Kozak, M., Bocianowski, J. and RybiNski, W. (2013). Note on the use of coefficient of variation for data from agricultural factorial experiments. Bulgarian Journal of Agricultural Science, 19(4): 644-646.
30. McDonald, J.H. (2014). Handbook of biological statistics (3rd Ed.). Sparky House Publishing, Baltimore, Maryland, USA.
31. FMANR (1990). Literature review on soil fertility investigation in Nigeria (in five volumes). Federal Ministry of Agriculture and Natural Resources, Abuja 281 pp.
32. Jones, M. J. (1973). The organic matter content of savannah soils of West Africa. Savannah Res. Bulletin, 186:42-53.
33. Enwezor, W.O., Udo, Ayotade, K.A., Adepelu, J.A. and Chude, V.O. (1990). Literature review on soil fertility investigation in Nigeria (In five volumes): Federal Ministry of Agriculture and Natural Resources; Lagos, Nigeria.
34. Balasubramanian, V. and L.A. Nnadi (1980). Crop residues management and soil productivity in savanna areas of Nigeria. P. 106-120. In Organic Recycling in Africa. FAO Soils Bull. 43. FAO, Rome.
35. Esu, I.E. (1991). Detailed soil survey of NIHORT farm at Bunkure, Kano State, Nigeria. Institute for Agricultural Research, Ahmadu Bello University Zaria, Nigeria. 72 pp. In: Kparmwang, T. Chude, V.O., Raji, B.A. and Odunze, A.C. Extractable 247 micronutrients in some soils developed on sandstone and shale in the Benue Valley, Nigeria. Nig. J. Soil Res. 1: 42-48
36. Nelson, D.W. and Sommers, L.E. (1982). Total carbon, organic carbon and orgarnic matter. InVan Reeuwijk, L.P. (Ed) Procedures for soil analysis (6th Edn). International Soil Reference and Information Center (ISRIC), Wageningen, The Netherlands, Food and Agriculture Organization of the United Nations. ISSN 0923-3792: No. 9.
37. Carling, D.E., Richle, N.E. and Johnson, D.R. (1978). Effect of VAM on nitrate reductase and nitrogenise activity in nodulation and non-nodulating soybean. Phytopathol., 68: 1590 – 1596
38. Kogbe, J.O.S. and Adediran, J.A. (2003). Influence of N, P and K application on the yield of maize in the savanna zone of Nigeria. African Journal of Biotechnology. 2: 345-349.
39. Lekberg, Y. and Koide, R.T. (2005). Arbuscular mycorrhizal fungi, rhizobia, available P and nodulation of groundnut (Arachis hypogea) in Zimbabwe. Agriculture Ecosystems & Environment. 110: 143-148.
40. Ibrahim, S.A. and Eleiwa, M.E. (2008). Response of groundnut (Arachis hypogaea L.) plants to foliar feeding with some organic manure extracts under different levels of NPK fertilizers. World Journals of Agricultural Sciences. 4: 140-148.
This article licensed under the Creative Commons Attribution 4.0 International License CC-BY 4.0., which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are properly credited.
