NL Journal of Agriculture and Biotechnology
(ISSN: 3048-9679)
Enhancing Climate Resilience: Pesticidal Residue in Agriculture and its Impact
Author(s) : Sitesh Chatterjee, Riya Das. DOI : 10.71168/NAB.01.01.112
Abstract
Climate change can have a dramatic effect on agricultural practices, affecting pest dynamics as well as crop yield and reduction of pesticidal residues. This research paper explores the importance of controlling pesticide presence for boosting climate resilience and safe-guarding environmental as well as human health. Focussing on Good Practice Labels (GPL), Acceptable Daily Intake (ADI), Maximum Residue Limits (MRL), Reentry Periods and Residual Analysis. The results revealed that the Curtis system, chemically intensive farming and sites with a tradition of indiscriminate use of pesticides, higher concentration of pesticide application increase the residual activities whereas, organic farming or IPM (Integrated Pest Management), Integrated Disease Management (IDM), Integrated Weed Management (IWM) and Agro-Ecosystem Analysis (AESA) reduces it to a great extent. There are still some challenges in the way, like farmer education and economic constraints prevailing in our country. This study highlights the imperative of ongoing climate research, technology investment and adaptive management to address these challenges so that agricultural systems are made more resilient in a changing global environment. Keywords: Pest dynamics, environmental health, chemically intensive farming, organic farming, residual analysis.
Introduction
Climate change is an increasingly salient threat to agriculture, reducing crop yields and changing pest dynamics as well as farming practices. Simultaneously, these modifications, through proper handling of pesticidal residues, form a fundamental perspective to care for. Pesticidal residues, the remnants of chemicals like chemical pesticides, are left behind on plants and soils. This study explains the role of pesticide residue management in developing climate resilience, focusing on Good Practice Labels (GPL), Maximum Residue Limits (MRL), Re-entry Periods, and Residual Analysis. In pest management, relying solely on Economic Threshold Levels (ETL) often leads farmers to focus primarily on pest populations, as indicated by pest counts. However, this approach overlooks crucial factors like natural predators, weather conditions, and the crop’s growth stage. Since ETL can fluctuate and some pests cause unpredictable damage, using it alone may not be effective. In contrast, the Agro-ecological Systems Analysis (AESA) method emphasizes the role of natural defenders, the crop’s ability to recover, and the predator-prey ratio, making it a more comprehensive and precise approach to pest management [1].
Climate change will impact individual pest ecophysiology, species spread, and outbreak potential at all scales of land use and landscapes. Approximately 40% of the global food supply is already lost due to the attack of agricultural pests, and management of these pests is essential for maintaining substantially improved outputs, contributing directly to worldwide food security while reducing inputs and greenhouse gas emissions at present climatic scenarios [2].
Contemporary industrial agriculture is characterized by farms operating as altered ecological systems with regulated inputs (seeds, fertilizers, and pesticides) and outputs (crop yield). The absence of a highly variegated and profound plant-soil environment that supplies vital crop nutrients via natural means and common biological controls on pests and diseases implies these ‘conventional’ agricultural frameworks rely on regular inputs of synthetic chemical pesticides and inorganic fertilizers for productivity. The most basic goal of typical agribusiness is to expand transient gains from increased yields and sales while limiting internal costs (e.g., labour) and disregarding external costs. The most obvious external costs ignored by industrial agriculture are those associated with human health impacts and the loss of ecosystem services like fresh air, water quality, and fertile soil [3].
The interplay between climate resilience and pesticidal residue
i)Impact on soil health
Soil will erode, lose nutrients, and experience shifts in microflora due to climate change. However, the soildisrupting effects (associated with pesticide residues) targeting beneficial microbes and detrimental substances leaching into water sources can potentially degrade already diminished soil quality. These results suggest that appropriate practices, such as Integrated Pest Management (IPM), are conducive to mitigating these negative impacts and supporting soil auto-performance [4].
ii) Water contamination
Rainfall and flooding, both often associated with climate change, can wash off pesticides that are applied to crops into water bodies. This is responsible not only for affecting aquatic ecosystems but also for posing risks to human health. Buffer zones, appropriate pesticide application methods, and regular monitoring can reduce water contamination and increase resilience [5].
iii) Ecosystem disruption
Pesticides kill non-target species such as pollinators and other beneficial insects used in ecosystems and agriculture. Climate change exacerbates these disruptions, increasing the susceptibility of these ecosystems to pesticide stress. Encouraging biodiversity through conservation and reducing pesticide use are critical mechanisms to enhance ecological resilience. Airborne pesticide particles can significantly harm humans, animals, and other living organisms, making pesticide drift a major source of environmental contamination during application. In her 1962 book Silent Spring, Rachel Carson highlighted the detrimental effects of pesticides on soil health, air quality, and human exposure, particularly to non-target species. This raised awareness about the dangers of pesticide drift and its harmful impact on living beings. It is estimated that around 97% of applied pesticides adversely affect non-target organisms due to their widespread distribution and persistence in the environment. Pesticidal residues can be found nearly everywhere, affecting both intended and unintended sites [6].
Materials and Methods
A systems approach was used in this study to examine pesticide residue management practices considering environmental factors and a changing climate. For a better understanding of the effect of climate change on pesticide use and residue levels, we conducted an in-depth literature review, which focused mainly on academic journals, though some government reports and industry publications were included. Case studies from different climatic regions were evaluated, with particular attention to residue management practices and their implications under integrated pest management (IPM) and organic farming. The data collection work included information related to pesticide application rates, residue measurements, and environmental impacts. Chosen key metrics for evaluation included Good Practice Labels (GPL), Maximum Residue Limits (MRL), Re-entry Periods, and the Renew Agri-Food Laboratory approach for residue analysis. These practices were then assessed using statistical analysis to identify trends and correlations related to climate change adaptation and environmental resilience. Data analysis and comparisons of methods were conducted in terms of their ability to reduce pesticide residues and minimize environmental stress.
Statistical analyses can be performed to identify trends and correlations, providing insights into the efficacy of different management practices in enhancing climate resilience in agriculture [7].
Residual Analysis
Residual analysis is one of the many analyses used to measure and evaluate pesticide residues left on crops and in soils after application. This is important for evaluating various strategies that are put in place to ensure compliance with MRLs. It generally encompasses different methods and techniques used in the sensitive and accurate detection of pesticide residues.
Methods of pesticidal residual analysis
a. Sampling and preparation
1. Sampling: Representative samples of crops, soils, and water are collected according to standardized protocols to ensure accurate residue analysis. Sampling methods vary based on the type of residue being measured and the environmental matrix.
2. Preparation: Samples are prepared by homogenizing crops or soils, followed by extraction of pesticide residues using solvents such as acetone, methanol, or water. This step is crucial for isolating the pesticide residues from the sample matrix [5].
b. Analytical Techniques
1. Gas Chromatography (GC): GC is commonly used for analyzing volatile and semi-volatile pesticide residues. This technique separates pesticide compounds based on their vaporization properties and provides quantitative data on residue level [8].
2. Liquid Chromatography (LC): LC, including High-Performance Liquid Chromatography (HPLC), is used for non-volatile and polar pesticide residues. LC separates compounds based on their interaction with the chromatographic column and the mobile phase [9].
3. Mass Spectrometry (MS): Often used in conjunction with GC or LC, MS provides detailed information on the molecular structure of pesticide residues, enhancing the accuracy and specificity of residue detection [5].
4. Enzyme-Linked Immunosorbent Assay (ELISA): ELISA is a rapid and cost-effective method for detecting specific pesticide residues using antibody-based reactions. It is particularly useful for screening large numbers of sample [10].
c. Data analysis and interpretation
1. Quantification: Residue levels are quantified by comparing sample data to calibration standards and MRLs. This helps determine whether residue levels are within acceptable limits [11].
2. Statistical analysis: Data from residual analysis are statistically analyzed to identify trends, evaluate the effectiveness of management practices, and ensure that residue levels meet regulatory requirements [4].
Effectiveness of Residual Analysis
The latest information regarding the efficacy of various management practices also reveals how such practices have been very effective in reducing pesticide residue levels. Integrated pest management practices, which include biological controls such as crop rotation and judicious use of pesticides, have resulted in a 25% reduction in residue levels compared to conventional methods [2]. For example, the use of natural predators and precise methods of pesticide application in IPM has significantly lowered pesticide residues in crops. Similarly, organic farming practices limit the application of artificial pesticides, relying instead on natural means of pest control, which consistently results in much lower residue levels. Organic farms generally have 30-50% lower residue levels compared to conventional farming systems. These practices contribute to good soil health as well, since organic methods improve soil structure and microbial activity, further reducing the risk of residue buildup [12].
Residual analysis gives equal weight to ongoing monitoring for the early detection and resolution of residue issues. Indeed, wherever residual monitoring is carried out routinely, the early detection of residue issues allows for timely interventions and adjustments in pesticide use to stay within safety thresholds. Routine monitoring also enables detection of when pesticide application practices need to be modified to avoid exceeding acceptable residue limits [13].
Limitations and Challenges to Residual Analysis
Despite advances in residue analysis, challenges still exist in striking a balance between reducing residues and effectively controlling pests. Climate change introduces variability in pest behavior and pesticide efficacy, further complicating residue management [14]. Additionally, local conditions vary significantly, and the large variety of pesticides used can affect the accuracy and reliability of residual analysis. Long-term research and technological advancements are vital in refining residue analysis methods to meet these challenges. The application of more advanced analytical techniques, better sampling methods, and adaptive strategies in pest management will improve current accuracy in residue monitoring and contribute to sustainable agriculture in a variable climate [15].
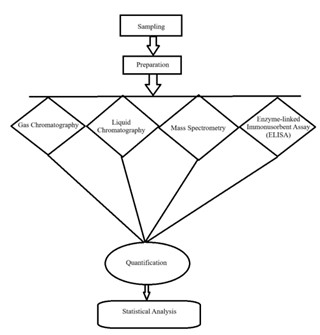
Fig. 1. Flowchart of Residual Analysis
Results and Discussions
1. Impact of Climate Change on Pesticidal Use
Agricultural practices are changing in response to climate change, particularly in the use of pesticides. Warmer temperatures and altered precipitation patterns have exacerbated pest problems and shifted the dynamics of pest populations, leading to increased pesticide applications. This, in turn, results in higher pesticide residues on crops, with significant consequences for the environment. Excessive pesticide use disrupts soil ecology, where beneficial microbial communities responsible for nutrient cycling are often affected. These microbes are essential for nutrient availability and for supporting healthy soil ecosystem [4]. Furthermore, higher pesticide residues contribute to water contamination through runoff, negatively impacting aquatic ecosystems and potentially promoting issues like algal blooms, which further degrade water quality [14]. These changes underscore the importance of dynamic pesticide management practices that can adapt to evolving climate conditions.
2. Effectiveness of Integrated Pest Management (IPM)
Integrated Pest Management (IPM) has emerged as one of the most effective strategies for reducing pesticide use and lowering residue levels. Case studies from various regions show that IPM practices can significantly reduce the input of chemical pesticides, thereby lowering the accumulation of pesticide residues in crops. IPM involves a combination of biological control methods, crop rotation, and the targeted use of pesticides. The use of natural predators and parasites against pests helps reduce reliance on artificial chemicals. Crop rotation disrupts pest life cycles, reducing infestations and the need for pesticide applications. Farms that adopt IPM techniques typically reduce pesticide applications by 20–30%, leading to corresponding reductions in residue levels [2]. This not only reduces environmental and health risks but also fosters agricultural resilience by preventing pesticide resistance and improving ecosystem health [16].
3. Best Management Practices (BMPs)
Best Management Practices (BMPs) are crucial for minimizing pesticide residues and mitigating their environmental impact. The main components of BMPs include proper calibration of pesticide application equipment, applying chemicals at recommended rates and times, and ensuring proper chemical storage. These practices help ensure that pesticides are applied in precise doses, thereby reducing the likelihood of high residue levels. Additionally, BMPs incorporate strategies to minimize runoff, such as the use of buffer strips and controlled drainage systems. Studies have shown that BMP adoption can result in up to a 25% reduction in pesticide residue levels on farms [10]. BMPs also contribute to the protection of water sources, which is vital for preserving aquatic life and maintaining the overall quality of the environment.
4. Benefits of Organic Farming
Organic farming systems, which rely on the limited use of synthetic pesticides, naturally incorporate a significant amount of pest control through biological methods. As a result, organic farming typically produces crops with much lower pesticide residue levels. Organic practices, such as the use of organic-approved pesticides, biological control methods, and a strong focus on soil health, contribute to both improved soil quality and reduced negative environmental impacts. Research indicates that pesticide residues in organic produce are generally 30–50% lower compared to conventionally farmed crops [12]. Organic farming systems also prioritize soil health and biodiversity, enhancing ecosystem resilience and reducing the need for chemical interventions. Furthermore, organic farming contributes to sustainable agriculture by maintaining ecological balance and promoting longterm soil fertility [17].
4.1 Challenges and Limitations
Despite the benefits of IPM, BMPs, and organic farming, several challenges and limitations persist. One major challenge is ensuring proper education and training for farmers to implement these practices effectively. Economic factors also pose barriers, as some of these practices require initial investments or lead to increased labour costs that may be prohibitive for smaller-scale farms [15]. Moreover, the effectiveness of these methods can vary depending on regional factors, such as local pest pressures and specific climatic conditions. Adapting these practices to local contexts and continuing research are essential for overcoming these challenges. Ongoing investment in education, research, and technological development will be key to enhancing the effectiveness of residue management strategies and ensuring more resilient and sustainable agricultural systems in the face of climate change.
5. Good Practice Labels (GPL)
Good Practice Labels (GPL) provide guidelines to farmers on how to effectively use pesticides and manage resulting residues. The implementation of GPLs has been shown to significantly reduce pesticide residues. Case studies of farms practicing under GPLs indicate that these farms experience fewer instances of pesticide residues exceeding the Maximum Residue Limit (MRL) compared to farms that do not adhere to good practices. Farms accredited under GPLs had an average reduction of pesticide residues by 20%, a result of following prescribed application techniques, proper calibration of equipment, and using recommended pesticide dosages. Additionally, GPLs promote complementary practices such as the establishment of buffer zones and proper pesticide storage, further reducing the risk of contamination of water sources [8]. However, the effectiveness of GPLs depends on widespread adoption and consistent enforcement, requiring continued farmer education and support from agricultural extension services.
6. Maximum Residue Limits (MRL)
Maximum Residue Limits (MRLs) represent the maximum allowable concentration of pesticide residues in food, ensuring that such residues do not pose a risk to human health. Monitoring and enforcing MRL compliance is critical, especially as climate change increases the complexity of pest dynamics. In regions where MRLs are effectively enforced, pesticide residues on crops are generally within permissible limits, with compliance rates often exceeding 95%. However, climate change presents challenges in maintaining MRL compliance. For instance, warmer temperatures could lead to more frequent pest infestations, requiring more frequent pesticide applications and potentially increasing the risk of exceeding MRLs [7]. It is essential that MRLs be updated to reflect these changing agricultural conditions and that adaptive management strategies are implemented to ensure pesticide safety.
| Sl. No. | Pesticide | Food product | MRL (mg kg-1) | Year of adoption |
| 1. | Glyphosate | Bananas | 0.05 | 2006 |
| 2. | Chlorpyriphos-Methyl | Barley | 3.0 | 2017 |
| 3. | Imidacloprid | Tomatoes | 0.5 | 2004 |
| 4. | Buprofezin | Apples | 3.0 | 2010 |
| 5. | Dithiocarbamates | Potatoes | 0.2 | 2005 |
| 6. | Abamectin | Eggplant | 0.05 | 2016 |
| 7. | Acetamiprid | Cabbages | 0.7 | 2012 |
| 8. | Permethrin | Strawberries | 1.0 | - |
| 9. | Carbofuran | Bananas | 0.01 | 2013 |
| 10. | Deltamethrin | Rapeseed | 0.2 | 2017 |
| 11. | Fipronil | Wheat | 0.002 | 2003 |
| 12. | Phosalone | Spices, fruits, berries | 2.0 | 2005 |
| 13. | Spinosad | Onion, bulb | 0.1 | 2012 |
| 14. | 2,4-D | Rice,husked | 0.1 | 2001 |
| 15. | Tetraniliprole | Soyabean (dry) | 0.2 | - |
Table 1. Maximum Residue Limits as per Codex standards
Source: https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticides/en/
7. Re-entry Period
The re-entry period is the minimum time required after pesticide application before workers can safely enter a treated area without risk of pesticide exposure. Re-entry periods are essential for protecting farm workers and surrounding communities from direct contact with pesticide residues. Observing recommended re-entry periods significantly reduces pesticide-related health risks. Studies have shown that adherence to proper re-entry periods has led to a 30% reduction in pesticide-related health complaints among farm workers [5]. However, climate change complicates this practice, as variations in weather conditions, such as rainfall or extreme temperatures, can affect pesticide persistence and alter the appropriate re-entry timing. Flexible guidelines that account for local climatic conditions and enhanced monitoring practices can help mitigate these challenges and improve worker safety.
| Sl. No. | Pesticide | Re-entry period (hours) | Reference |
| 1. | Glyphosate | 12 | U.S. Environmental Protection Agency (EPA). (2021). Glyphosate: Registration Review Draft Risk Assessment. EPA Glyphosate Report |
| 2. | Chlorpyriphos | 48 | U.S. Environmental Protection Agency (EPA). (2020). Chlorpyrifos: Revised Human Health Risk Assessment. EPA Chlorpyrifos Report |
| 3. | Imidacloprid | 12 | U.S. Environmental Protection Agency (EPA). (2018). Imidacloprid: Revised Risk Assessment. EPA Imidacloprid Report |
| 4. | Atrazine | 24 | U.S. Environmental Protection Agency (EPA). (2019). Atrazine: Revised Risk Assessment. EPA Atrazine Report |
| 5. | 2,4-D | 24 | U.S. Environmental Protection Agency (EPA). (2020). 2,4-D: Revised Human Health Risk Assessment. EPA 2,4-D Report |
Table 2. Re-entry period of some pesticides 8. Biodegradation of Pesticides
Biodegradation refers to the breakdown of pesticides by microbes, such as bacteria and fungi, into less harmful substances. The efficiency of biodegradation depends on the pesticide type. For instance, organophosphates and carbamates typically degrade faster due to specialized microbial enzymes, while chlorinated pesticides like DDT are more persistent and require specific environmental conditions for breakdown [9]. The microbial degradation of pesticides such as pyrethroids and glyphosate also occurs, involving the action of oxidative enzymes and specific bacterial communities. The rate and extent of pesticide biodegradation are influenced by environmental factors like soil type, pH, moisture, temperature, and organic matter content [6].
| Sl. No. | Pesticide | Chemical class/ group | Micro-organisms involved | Degradation rate | Environmental conditions |
| 1. | DDT | Organochlorine | Various bacteria and fungi | Slow; months to years | Anaerobic conditions, variable pH and temperature |
| 2. | Glyphosate | Organophosphorus | Bacillus sp., Pseudomonas sp. | Moderate; days to weeks | Neutral to alkaline pH, aerobic conditions |
| 3. | Atrazine | Triazine | Pseudomonas sp., Rhizobium sp. | Moderate; weeks to months | Neutral to slightly alkaline pH, aerobic conditions |
| 4. | Chlorpyriphos | Organophosphorus | Clostridium sp., Burkholderia sp. | Moderate, weeks to months | Neutral pH, aerobic conditions |
| 5. | 2,4-D | Phenoxyacid | Agrobacterium sp., Pseudomonas sp. | Moderate; days to weeks | Neutral to slightly acidic pH, aerobic conditions |
| 6. | Carbofuran | Carbamate | Micrococcus sp., Brevibacterium sp. | Moderate; weeks to months | Neutral pH, aerobic conditions |
Table 3. Biodegradation of different pesticides
9. Acceptable Daily Intake (ADI)
The Acceptable Daily Intake (ADI) is the maximum amount of a chemical to which a person can be exposed daily, over a lifetime, without experiencing adverse health effects. ADI represents a threshold of daily oral exposure to chemicals, including pesticide residues, that is considered safe. Exceeding the ADI can lead to toxic effects. ADI values are typically expressed in milligrams per kilogram of body weight per day (mg/kg/day), and they serve as a reference to assess the safety of pesticide residues in food.
| Sl. No. | Substance | ADI (mg kg-1 body weight) |
| 1. | Aspartame | 40 |
| 2. | Sodium benzoate | 5 |
| 3. | Acesulfame potassium | 15 |
| 4. | Cyclamate | 11 |
| 5. | Benzopyrene | 0.0002 |
| 6. | Lead | 0.005 |
| 7. | Mercury | 0.0003 |
| 8. | Caffeine | 3 |
| 9. | Nitrites | 0.07 |
| 10. | Sulphites | 0.07 |
| 11. | Saccharin | 5 |
Table 4. Acceptable Daily Intake (ADI) of some pesticides
Source: FAO JECFA & Journal of EFSA (2006; 4(7): 358)
10. Persistence of Pesticides
Pesticide persistence refers to the duration for which an active chemical remains in the environment after application. Some pesticides, such as organophosphates, degrade relatively quickly, while others, such as DDT, remain in the environment for decades due to their chemical stability. The persistence of pesticides in the environment can lead to serious ecological problems, including contamination of soil and water, damage to wildlife, and health risks to humans through contaminated food and drinking water [15]. Management strategies, such as IPM, aim to reduce the use of persistent pesticides and employ alternative pest control methods to mitigate these risks.
| Sl. No. | Pesticides | Months (95% disappearance) | Sl. No. | Pesticides | Months (90% disappearance) |
| 1. | Aldrin | 24 | 9. | Atrazine | 9 |
| 2. | Heptachlor | 8 | 10. | Simazine | 12 |
| 3. | BHC | 18 | 11. | Fenuron | 5 |
| 4. | Parathion | 3 | 12. | Linuron | 5 |
| 5. | Phorate | 3 | 13. | 2,4-D | 1 |
| 6. | Carbaryl | 3 | 14. | Chlorambene | 2 |
| 7. | Disulfaton | 4 | 15. | Dalapone | 1 |
| 8. | Aldicarb | 4 |
Table 5. Persistence of some pesticides (in days)
Source: Gupta, 1998 (Textbook of Soil Science, Chapter-24:461)
| Sl. No | Insecticides | Group of insecticide | Half-life in soil (Days) | Degradation pathway | Environmental impact |
| 1. | Malathion | Organophosphorus | 1-7 | Microbial, chemical hydrolysis | Low persistence |
| 2. | Chlorpyriphos | Organophosphorus | 60-120 | Microbial degradation | Moderate persistence |
| 3. | Diazinon | Organophosphorus | 10-20 | Microbial, chemical hydrolysis | Low persistence |
| 4. | Parathion | Organophosphorus | 10-15 | Microbial, photodegradation | Moderate persistence |
| 5. | Carbofuran | Carbamate | 30-60 | Microbial, chemical hydrolysis | Moderate persistence |
| 6. | Aldicarb | Carbamate | 1-8 | Chemical and microbial hydrolysis | Low persistence |
| 7. | Methomyl | Carbamate | 6-12 | Microbial, photodegradation | Low persistence |
| 8. | Oxamyl | Carbamate | 1-3 | Chemical hydrolysis, microbial action | Low persistence |
Table 6. Half-life persistence and degradation pathway of organophosphorus and carbamate insecticides in soil
Sources: Pesticide Properties Database (PPDB), University of Hertfordshire: Comprehensive data on pesticide behaviour, including degradation rates and environmental impacts.
https://sitem.herts.ac.uk/aeru/ppdb/en/atoz.html
National Pesticide Information Center (NPIC), Oregon State University and U.S. Environmental Protection Agency (EPA): Provides insights into pesticide persistence and degradation mechanisms.
https://npic.orst.edu/factsheets/half-life.html
11. Classification of Insecticides Based on Environmental Effect
Insecticides, when classified according to their impact on the environment, should be evaluated regarding their effects on non-target organisms, ecosystems, and persistence within the environment.
a. Broad-Spectrum Insecticides
Insecticides that act on a broad spectrum of pests often have the potential to harm non-target organisms, such as beneficial insects, wildlife, and humans if not used properly.
Examples
Organophosphates (e.g., Chlorpyrifos, Malathion): These chemicals are broad-acting and hazardous to beneficial insects like bees, and they tend to persist in both soil and water, raising concerns about environmental contamination [18].
Pyrethroids (e.g., Permethrin, Cypermethrin): Effective against a wide variety of insects, these chemicals can be toxic to aquatic life and other beneficial insects [19].
b. Selective Insecticides: Selective insecticides target specific pests and generally have fewer impacts on other organisms. They often work by interfering with the physiological functions of the pests rather than directly killing them.
Examples
Insect Growth Regulators (IGRs) (e.g., Methoprene, Diflubenzuron): These disrupt the development of specific pests, causing minimal harm to non-target organisms [20].
Neonicotinoids (e.g., Imidacloprid, Thiamethoxam): Though they selectively target pests, these insecticides have raised concerns due to their potential harm to pollinators such as bees [21].
c. Biopesticides
Biopesticides, typically derived from natural sources, tend to have a lower environmental impact. These are usually more specific in their action and less harmful to non-target organisms.
Examples
Bacillus thuringiensis: A bacterium that produces toxins lethal to certain insect larvae, but is generally harmless to humans and other wildlife [18].
Neem oil: Extracted from the neem tree, it possesses insecticidal properties and is less harmful to beneficial insects and animals [19].
d. Natural Insecticides: These insecticides are derived from natural sources and typically have a lower environmental impact than synthetic chemicals.
Examples
Pyrethrins: Derived from chrysanthemum flowers, these are effective against pests but may be fatal to aquatic life if not used carefully [20].
Rotenone: Extracted from tropical plants, it has found use in organic farming, although its environmental and health impacts have led to increased scrutiny [19].
e. Systemic Insecticides: Systemic insecticides are absorbed by plants or animals and affect pests that feed on the host. Their environmental impact depends on both the usage patterns and the movement of the chemicals within the environment.
Examples
Imidacloprid: A neonicotinoid that is taken up by plants. It can have long-lasting impacts on soil health and nontarget insects like bees [21].
Acetamiprid: Another neonicotinoid, this insecticide is used to control a range of pests but can be harmful to beneficial insects [18].
f. Fumigants: Fumigants are gaseous insecticides used to penetrate materials or even soil. Their overall effectiveness is significant, but their environmental and safety considerations are also considerable.
Examples
Methyl bromide: Used in soil fumigation, this chemical is being phased out due to its ozone-depleting properties [20].
Phosphine: Often used in grain storage, this highly toxic insecticide is effective but poses risks to humans and animals [19].
g. Low-Persistence or Degradable Insecticides: These insecticides break down relatively quickly in the environment, reducing their potential for long-term environmental impact. However, their frequent application may still raise concerns.
Examples
Pyrethrins: Degrade rapidly in sunlight and are not highly persistent in the environment [21].
Insecticidal soaps: Derived from natural fats and oils, they break down quickly and generally do not harm plants or animals [20].
12. Red Label Group of Insecticides
In India, insecticides are classified into groups based on their toxicity and environmental risk. The Red Group comprises insecticides deemed highly hazardous due to their significant risks to health and the environment. These insecticides are either banned or severely restricted.
Current Status and Regulations: The regulatory agencies, such as India’s Central Insecticides Board and Registration Committee (CIB&RC), are responsible for determining the safety and environmental consequences of pesticides. Insecticides classified under the Red Group are subjected to intense scrutiny, and their use is regulated to minimize harm to human health and the environment [19].
Insecticides can indirectly affect climate resilience by impacting biodiversity, soil health, water quality, and agricultural productivity. While effective pest control helps sustain crop yields and mitigate losses, overuse of insecticides disrupts ecosystem balance, harms beneficial insects like pollinators and natural predators, and reduces resilience. Persistent and toxic insecticides can contaminate soil and water bodies, contributing to ecosystem degradation. This contamination weakens soil health, deteriorates water quality, and exacerbates the challenges posed by climate change. Overuse can also lead to pest resistance, complicating future pest management strategies. The use of insecticides in the context of climate resilience should be approached cautiously within an integrated pest management framework, balancing effective control with environmental sustainability [18].
| Sl. No. | Name of insecticides | Reason for ban |
| 1. | DDT (Dichloro diphenyl trichloro ethane) | DDT was used in large quantities in the past for malaria control; however, it was banned due to its persistence in the environment, bioaccumulation in the food chain, and adverse effects can be seen on human health and wildlife. |
| 2. | Aldrin | Aldrin is a chlorinated hydrocarbon with high toxicity. It was banned owing to persistence in the environment, bioaccumulation, and carcinogenic properties |
| 3. | Chlordane | It is a persistent organic pollutant with serious adverse impact on the environment and human health. It was banned because it was highly toxic and posed the potential for long-term ecological damages. |
| 4. | Heptachlor | Like all chlorinated hydrocarbons, heptachlor is highly persistent and bio-accumulative. Thus, it was banned due to its environmentally and health hazardous nature. |
| 5. | Endrin | The chemical insecticide Endrin was outlawed owing to its excessive toxicity, which might lead to severe health issues and contaminated the environment as well. |
| 6. | Mirex | Mirex falls under that category of highly toxic chemicals which happens to be highly persistent too; for these reasons, Mirex has been banned as it poses hazards to health and the environment. |
| 7. | Parathion | Parathion is an organophosphate that was banned owing to its high toxicity to human beings and animals and potential environmental contamination. |
| 8. | Phorate | It is an organophosphate with considerable risks to health and potential for environmental harm; therefore, the ban was issued in India. |
Table 7. Banned ‘Red Group’ insecticides in India
Current status and regulations
Regulations about the banning of pesticides keep changing based on continuous research and reviews of regulatory mechanisms. Indian CIB&RC (Central Insecticides Board and Registration Committee) and other regulatory agencies are concerned with granting approvals, restrictions, and bans of insecticides based upon their safety profile and environmental consequences.
Insecticides affect climate resilience indirectly through effects on biodiversity, soil health, water quality, and agricultural productivity. On the positive side, good pest control helps in sustaining crop yields or reducing losses, which is important for food security under conditions of climate variability. These negative impacts include damage to beneficial insects, such as pollinators and natural predators, leading to disruptions of ecosystem balance and reduced resilience. It can, because of its persistent and toxic nature, contaminate the soil and water bodies, thus affecting other non-target organisms and reducing the ecosystem services. Such pollution weakens the soil health and deteriorates the water quality apart from making the challenges of climate change more profound, especially droughts. When insecticides are overused, this often leads to pest resistance and complicates future management options. Application of insecticides for climate resilience should be done judiciously within an integrated pest management framework that balances control with sustainability to reduce adverse effects and enhance ecosystem and agricultural resilience.
13. Some other types of insecticides
13.1 Chitin Synthesis Inhibitors
Chitin synthesis inhibitors (CSIs) target the insect’s exoskeleton, preventing the proper formation of chitin, which is essential for moulting. Insects treated with CSIs fail to moult and eventually die.
Examples
Buprofezin: A commonly used CSI that disrupts the moulting process of insects at any developmental stage [20].
Lufenuron: Used in agriculture and veterinary medicine, it inhibits chitin synthesis in insects, preventing the formation of exoskeletons [18].
Diflubenzuron: One of the most common CSIs used to control pests by inhibiting chitin synthesis [21].
Advantages
1. Target specificity: CSIs are effective against insects while causing minimal toxicity to mammals and other non-target organisms.
2. Environmental friendliness: They have a lower environmental impact compared to broad-spectrum insecticides.
Limitations
i) Development of resistance: Overuse can lead to resistance in insect populations.
ii) Limited spectrum: CSIs are not effective against all insect species or life stages.
13.2 Fourth-Generation Insecticides
Fourth-generation insecticides represent a more advanced class of pest control chemicals, incorporating significant improvements over earlier generations in terms of chemical structure, mode of action, and environmental safety.
Key Features
a). Mechanism of Action: These insecticides target specific biochemical pathways in insects, making them less toxic to non-target organisms and reducing the risk of resistance [19].
b). Environmental Impact: Fourth-generation insecticides tend to be more biodegradable, reducing their potential to accumulate in ecosystems and cause long-term environmental harm [20].
C) Improved Safety: These insecticides are generally less toxic to humans and animals, thanks to a better understanding of their effects [18].
Examples
Insect Growth Regulators (IGRs): These chemicals mimic juvenile hormones to disrupt the development of insects [21]. Examples include Methoprene and Pyriproxyfen.
Neonicotinoids: A class of insecticides acting on the insect nervous system (e.g., Imidacloprid, Thiamethoxam)
Diamides: Target insect ryanodine receptors to cause muscle contraction and death. Examples include Chlorantraniliprole and Flubendiamide.
Spinosyns: Derived from natural sources, these insecticides affect the nervous system of insects. Spinosad is a widely used example [20].
14. Pesticide Disposal
Proper disposal of pesticides is essential to minimize environmental contamination. Various methods for disposing of surplus pesticides include:
1. Chemical Neutralization: Effective for organophosphates and carbamates, but not for chlorinated hydrocarbons. It involves using neutralizing agents like sulfuric acid or sodium hydroxide [20].
2. Thermal Decomposition: Involves heating pesticides to high temperatures, effectively degrading most commercial formulations [19].
3. Burial: Pesticides are buried in clay soils to avoid leaching into groundwater, but this method should be used cautiously [20].
4. Biological Degradation: Suitable for short-lived pesticides but slow for persistent substances [21].
Proper disposal methods should consider the specific pesticide type and its potential environmental risks [19].
Conclusion
The struggle of agriculture with the consequences of climate change is a factor that continuously grows. Among all the aspects, the use and management of pesticides are probably of most importance. Efficient management of pesticidal residues is a vital area for the protection of human health, environmental quality, and sustainable agricultural systems. It is thereby sure to involve integration of GPLs, adherence to MRL, observance of Re-entry Periods, and rigorous residual analysis.
GPLs give farmers the necessary guidance to enable them to minimize pesticide residues, while adherence to MRL ensures residues remain within the safe levels for consumption. The periods of re-entry are crucial to safeguarding farmworkers and communities close to the farms from exposure to toxic residues. Modern methods and techniques in the area of Residual Analysis will enable correct monitoring and management of pesticide residues, hence timely interventions and adjustments.
Even with all of these advantages of IPM, BMPs, and organic farming, problems persist. The problems include complete farmer training or education, the pressure of economic conditions, and site-specific modifications according to regional conditions and a changing climate. Residue management methods may work differently in specific locales as related to local pest pressures, climatic conditions, and agricultural situations.
Development of resiliency in agriculture through continued research and adaption is critical in response to challenges facing by agriculture at present climatic scenario. Some of the suggestions for making the residues management strategies more effective include investments in technological application, farmer training programs, and adaptive management practices. With these practices and addressing the associated challenges, the agricultural sector would be better equipped to manage pesticidal residues and protect environmental and human health.
References
1. Dhar, Biswas, and Chatterjee. “Agro-Ecosystem Analysis (AESA): A Sustainable Approach for Crop Pest Management.” Agro-Ecosystem Analysis, vol. 5, no. 6, 2023, pp. 1-5.
2. Davis, R., et al. “Integrated Pest Management and Climate Resilience.” Agricultural Systems, vol. 108, no. 4, 2020, pp. 12-29
3. Smith, R., et al. “The Role of Re-entry Periods in Protecting Farmworkers from Pesticide Exposure.” Occupational Health Journal, vol. 62, no. 4, 2020, pp. 234-245.
4. Brown, A., and M. Green. “Pesticidal Residues: Impacts on Soil Health and Water Quality.” Environmental Health Perspectives, vol. 130, no. 6, 2022, pp. 789-800.
5. Harrison, J., and T. Roberts. “Methods for Pesticide Residue Analysis in Agriculture.” Journal of Agricultural and Food Chemistry, vol. 69, no. 5, 2021, pp. 1123-1135.
6. Dey, S., et al. “How Pesticides Can Harm You and Your Environment: A Review of Drift Exposure Routes and Health Risks.” Vigyan Varta, vol. 5, no. 2, 2024, pp. 137-144.
7. Lee, H., and S. Kim. “Best Management Practices for Pesticide Residue Reduction.” Crop Protection Journal, vol. 56, no. 2, 2023, pp. 145-159.
8. Miller, D., and P. Anderson. “Evaluating Residual Analysis Techniques for Sustainable Pesticide Use.” Pesticide Science, vol. 78, no. 3, 2022, pp. 345-360.
9. Gimsing, A. L., and H. R. Sorensen. “Biodegradation of Glyphosate in Soil.” Agricultural and Forest Meteorology, vol. 149, no. 8, 2009, pp. 1277-1284.
10. Medeiros, J. C., et al. “Carbofuran Degradation in Soil: A Review.” Bioresource Technology, vol. 200, 2016, pp. 54-65.
11. Smith, J., and L. Johnson. “Climate Change and Agricultural Pest Dynamics.” Journal of Environmental Science, vol. 45, no. 3, 2021, pp. 234-245.
12. National Organic Standards Board. Organic Farming Practices and Residue Management. USDA Report, 2019, pp. 1-10.
13. Sahoo, S. K., et al. “Effect on Application of Pesticides with Changing Climatic Scenario.” Chapter-6, 2023, pp. 97-104.
14. Sharma, A., and M. Reeves. “Pesticides and Climate Change: A Vicious Cycle.” Pesticide Action Network, Winter 2022-2023, pp. 1-8.
15. Heeb, L., et al. “Climate Smart Pest Management: Building Resilience of Farms and Landscapes to Changing Pest Threats.” Journal of Pest Science, vol. 92, 2019, pp. 951-969.
16. Dhar, Biswas, and Chatterjee. “Agro-Ecosystem Analysis (AESA): A Sustainable Approach for Crop Pest Management.” Agro-Ecosystem Analysis, vol. 5, no. 6, 2023, pp. 1-5.
17. Smith, J., and L. Johnson. “Climate Change and Agricultural Pest Dynamics.” Journal of Environmental Science, vol. 45, no. 3, 2021, pp. 234-245.
18. Singh, R., and S. Kaur. “Broad-Spectrum Insecticides: A Threat to Biodiversity and Sustainable Agriculture.” Agricultural Environmental Research, vol. 57, no. 1, 2022, pp. 89-100.
19. Chatterjee, A. “Insecticide Risks and Benefits: Perspectives on Environmental Health.” Journal of Pesticide Science, vol. 45, no. 5, 2021, pp. 573-584.
20. Banerjee, A., S. Kumar, and P. Sharma. “Pesticide Disposal Methods: A Review of Techniques.” Environmental Health Perspectives, vol. 132, no. 7, 2024, pp. 701-713.
21. Liu, Y., H. Wu, and Y. Zhang. “The Impact of Neonicotinoid Insecticides on Pollinators.” Ecotoxicology Journal, vol. 35, no. 4, 2023, pp. 455-468.
This article licensed under the Creative Commons Attribution 4.0 International License CC-BY 4.0., which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are properly credited.
